A thermodynamic system is a confined space of matter (e.g. gas) within which thermodynamic processes take place. The system boundary separates the system from the environment.
Definition of the term thermodynamic system
In order to describe thermodynamic processes, one must first define what exactly is to be described. This leads to the term thermodynamic system, within which thermodynamic processes take place and are described. In general, a system is a confined space within which matter (e.g. gas) is in different states. This system is described among other things by the thermodynamic state quantities such as pressure, volume, temperature and mass.
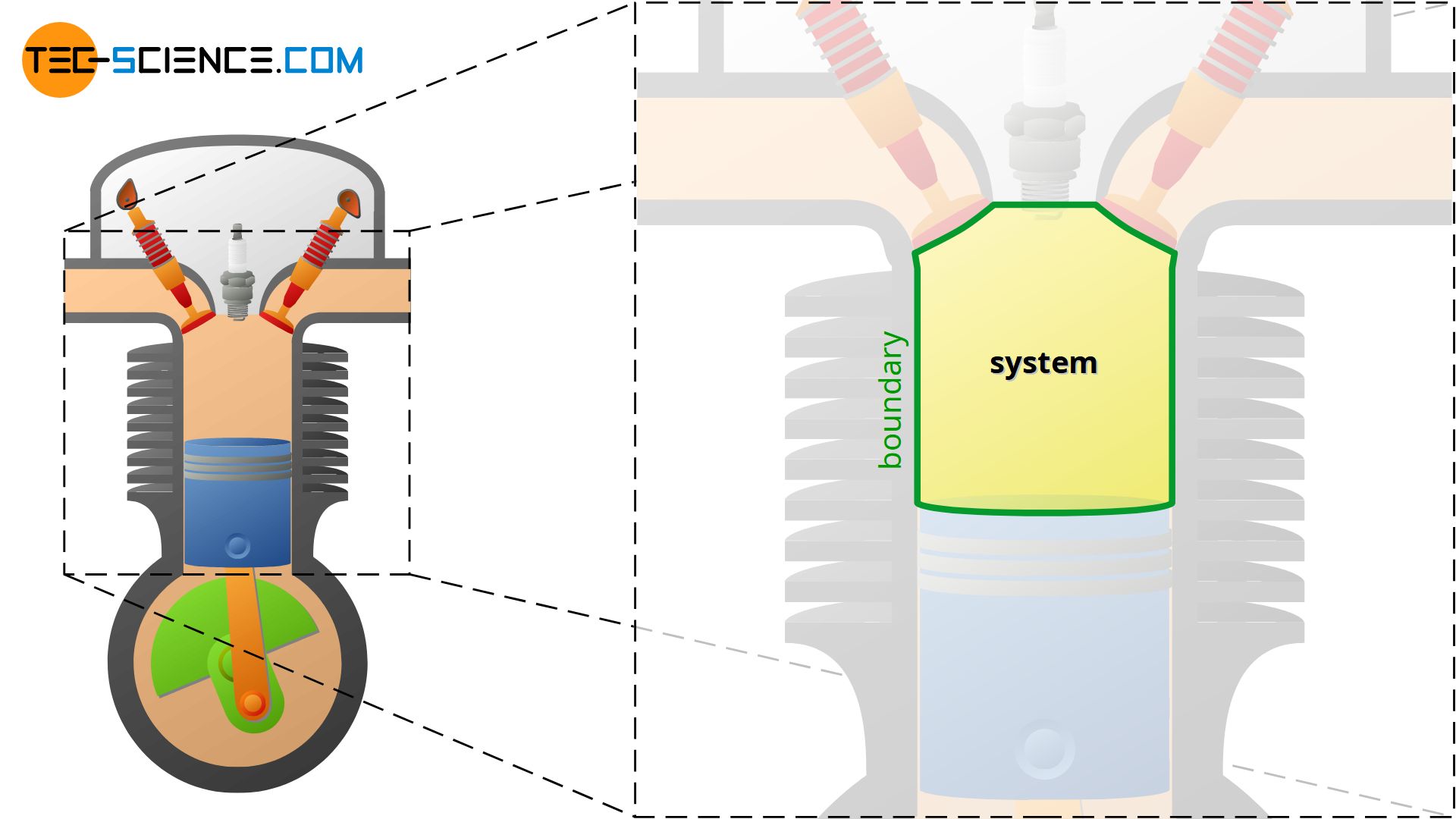
If, for example, the process in a internal combustion engine is to be described, the space in the cylinder is the thermodynamic system. The changes of state of the confined gas through heating, cooling, expansion and compression are described within this system.
In this case the system is defined by the inner surface of the cylinder and the surface of the piston. Such a boundary of the contained gas is also called system boundary. The system boundary thus separates the actual thermodynamic system from the surroundings. As the example of the combustion engine shows, the system boundary or the thermodynamic system does not necessarily have to be rigid, but can increase (expansion) or decrease (compression) during a thermodynamic process!
A thermodynamic system is a confined space of matter (gas) within which thermodynamic processes take place. The system boundary separates the system from the environment.
Change of the state of a system by an exchange of energy
In general, a system can exchange energy as heat and as work with the surroundings across the system boundary, i.e. energy can be transferred from the system to the surroundings (cooling or expansion) or energy can be transferred from the surroundings to the gas (heating or compression). An exchange of matter is also possible between the system and the surroundings. The exchange of energy or matter changes the state of the thermodynamic system.
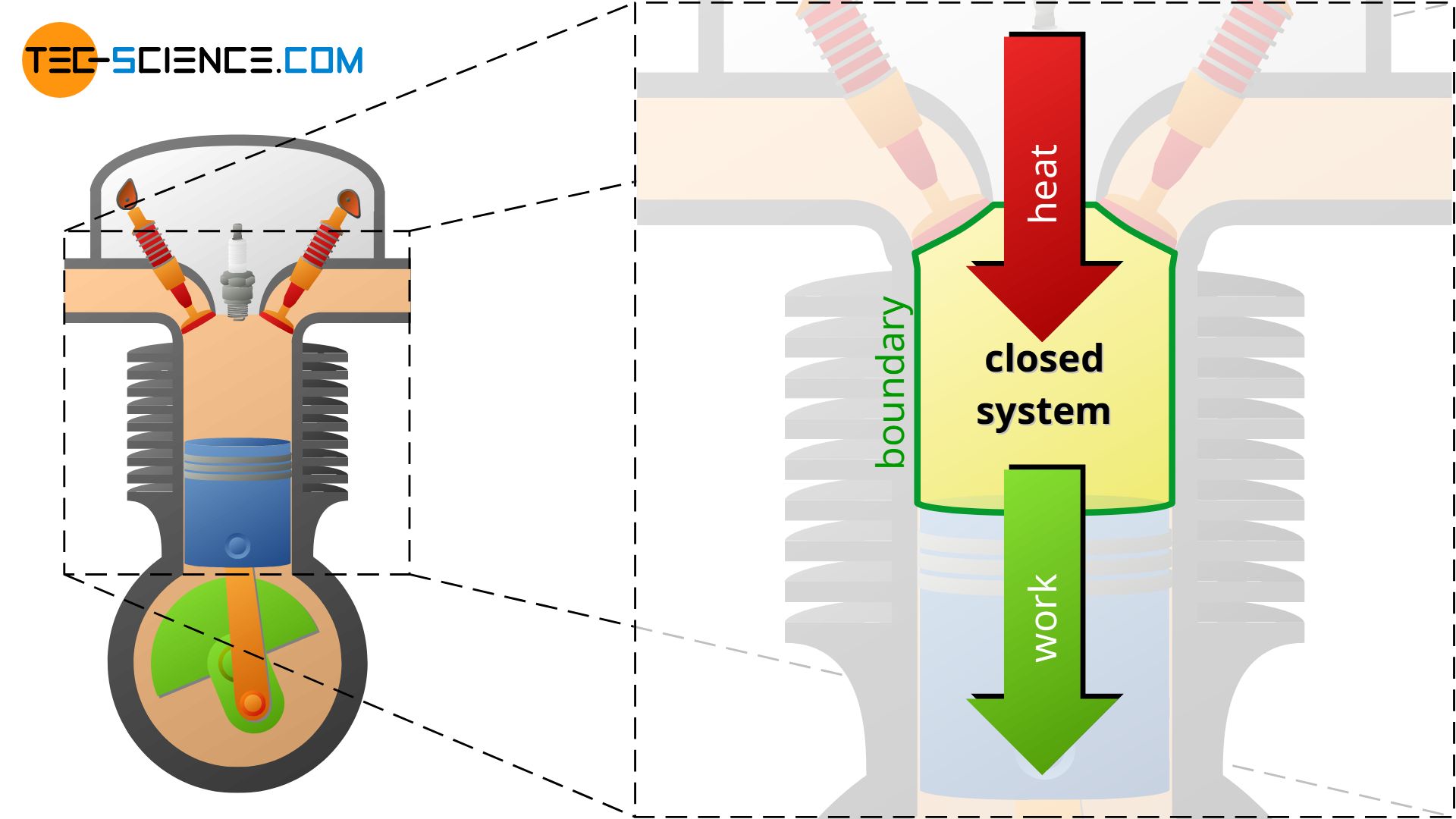
This will be illustrated using the example of a 4-stroke internal combustion engine. Heat is supplied to the gaseous fuel-air mixture by combustion of the fuel. This leads to an increase in temperature and pressure (change in the state of the system due to the transfer of heat). Then the power stroke takes place due to the expanding gases. The hot gases push the piston down due to the high pressure. Thereby the gas transfers work across the system boundary to the surroundings, i.e. mechanical energy is transferred from the gas to the connecting rod and then to the crankshaft. Pressure and temperature decrease during the expansion (change in the state of the system due to the transfer of work).
Importance of the boundary using an engine as an example
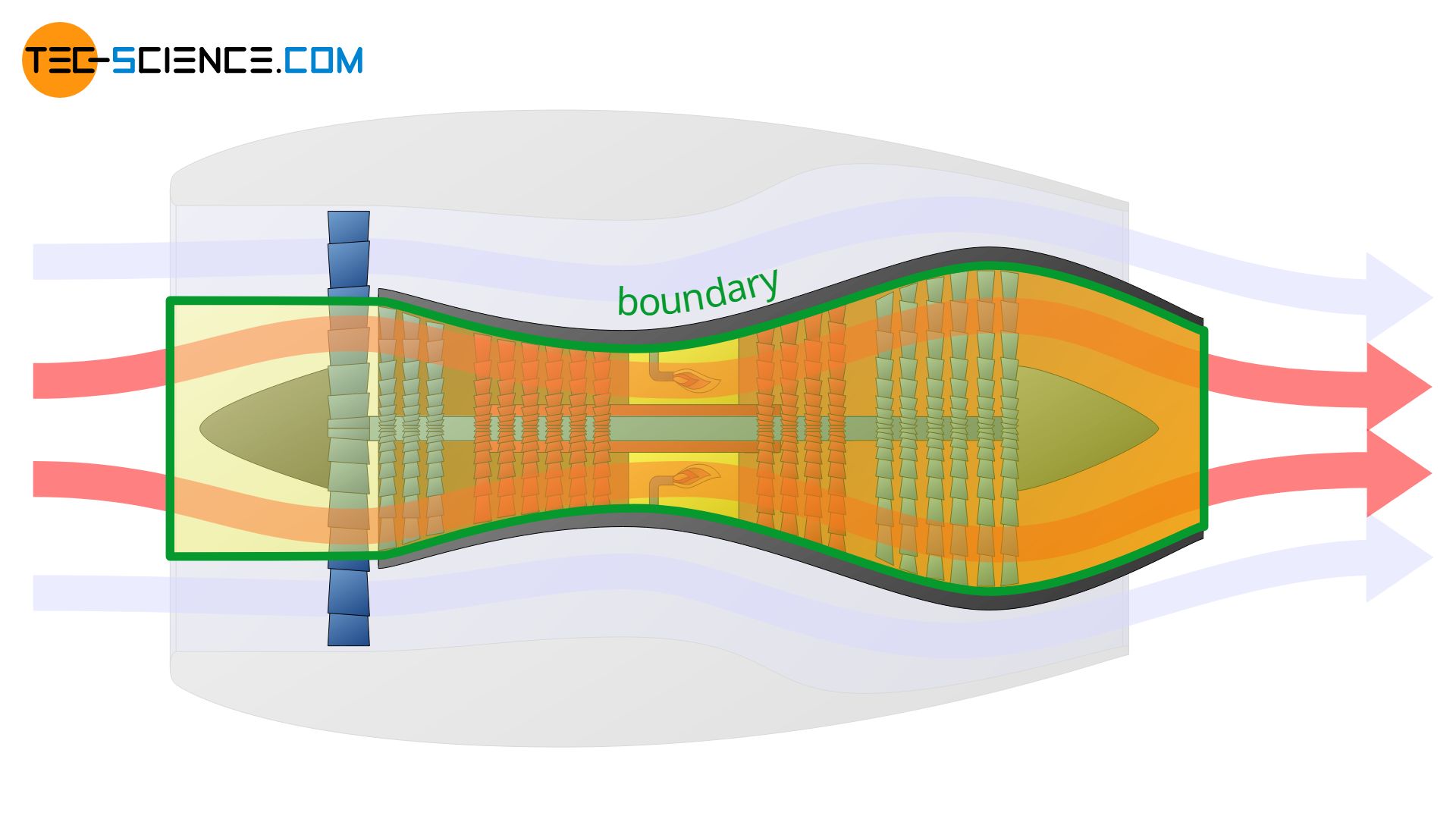
The concept of system or boundary is very abstract. However, the definition of the boundary is crucial when it comes to the description of thermodynamic processes. Depending on the definition, completely different thermodynamic processes may be described. The example of a turbofan engine is used to illustrate this.
In a turbofan engine, the intake air is first divided into two flows: a core flow and a bypass flow, which is routed around the turbine via a bypass. The core flow is routed through the core engine and the combustion chamber and provides the drive for the actual turbine, while the bypass flow generates 85 % of the entire thrust (only 15 % of the thrust is generated by the core flow).
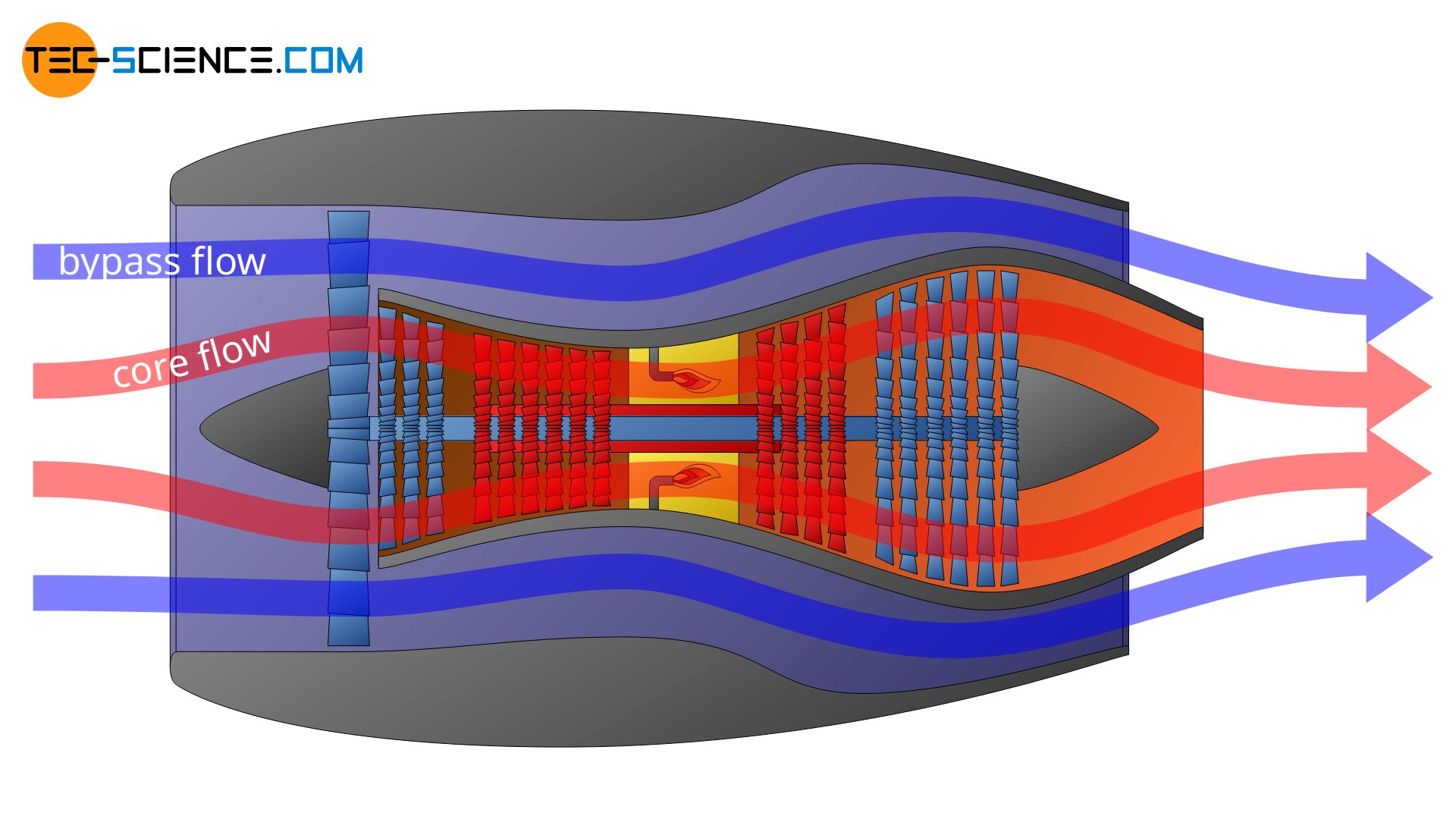
Depending on how the system boundary is now set, very different thermodynamic processes will be described. In the first of the cases shown below, only the change of state of the bypass flow is described. In the second case only the thermodynamic processes taking place in the core flow are described.
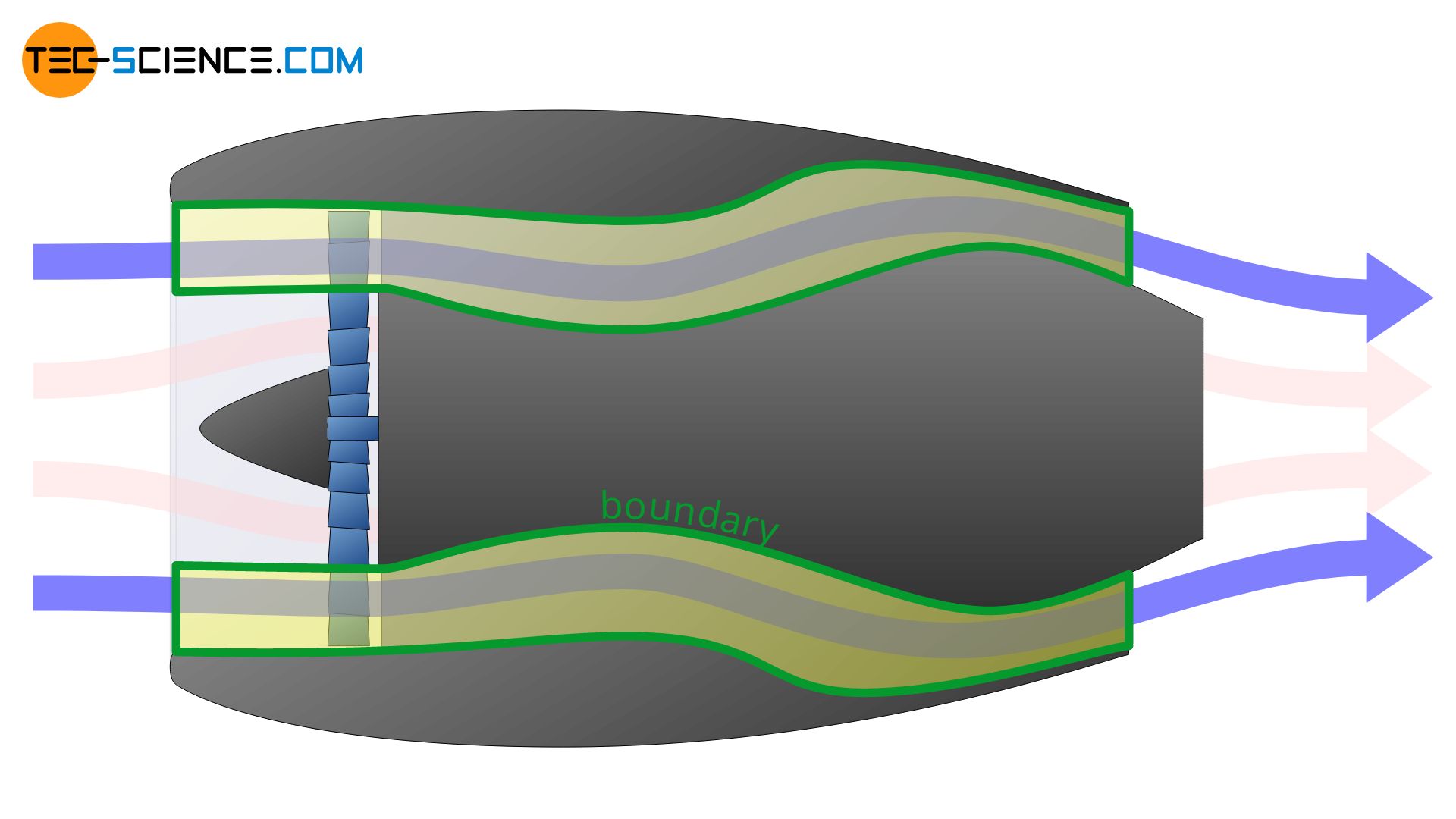
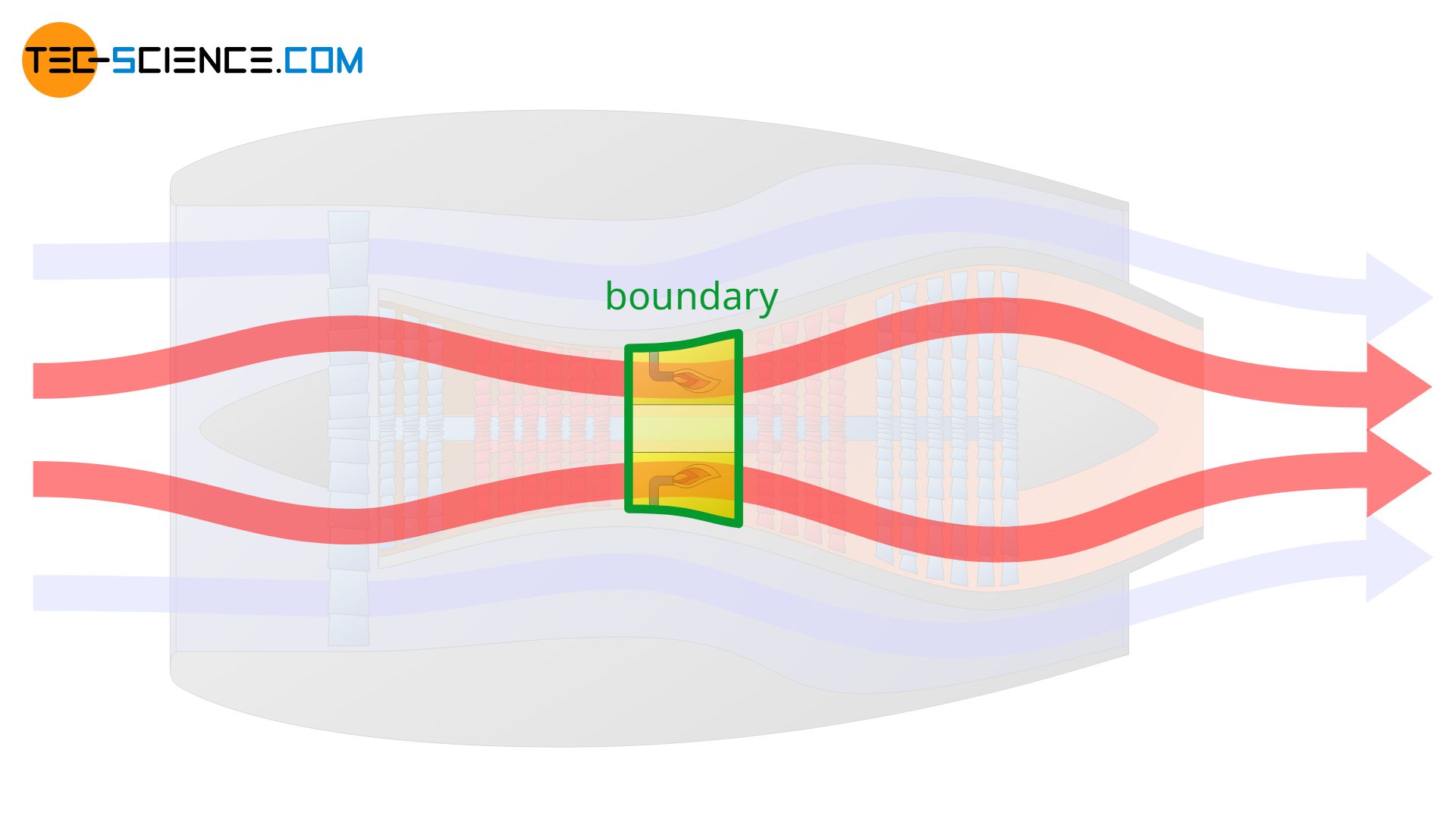
However, the system boundary can also be set only around the combustion chamber. In this case, only the thermodynamic processes within the combustion chamber are described. It is obvious that the definition of the system boundary is crucial for the whole process to be described.
Types of thermodynamic systems
Different thermodynamic systems can be distinguished depending on the permeabilities of the system boundaries. These are discussed in more detail in the following sections.
Open thermodynamic systems
An open system (flow system) can transfer energy as work and as heat across the boundary to the surrounding and vice versa, i.e. work and heat can also be transferred from the surrounding to the system. Furthermore, matter can be transferred into the system or out of the system. Thus, the boundary of an open system is permeable for work, heat and matter.
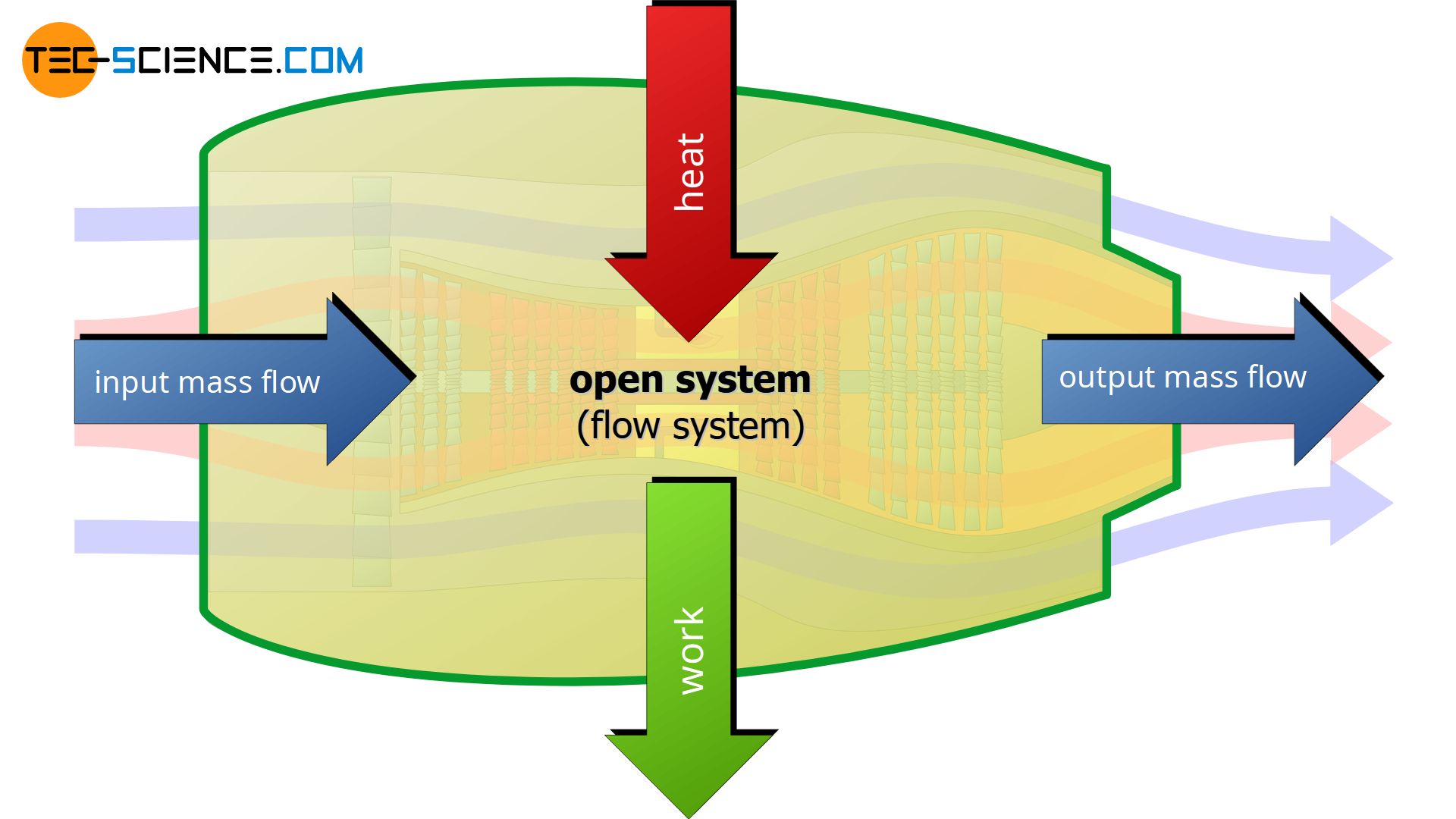
A typical example of an open system is a turbine where the system boundary is the turbine housing. The turbine sucks in air through the boundary, which results in a mass flow. The air is then compressed and mixed with fuel and heated (combustion of fuel), which then leads to a strong expansion of the gas. The expansion of the gas sets/keeps the turbine shaft in motion. The burned fuel-air-mixture then leaves the turbine through the boundary. Part of the work done by the expansion is used to drive the compressor. The excess work (which is generated by the heat of combustion) provides the thrust of the turbine. This thrust is the actual work that crosses the boundary.
Closed thermodynamic systems
In a closed system, energy as work and as heat can be exchanged across the system boundaries, but not matter. Unlike an open system, a closed system is therefore only permeable to work and heat and impermeable to matter.
The cylinder of a four-stroke engine during the compression stroke and power stroke, for example, is such a closed system. The valves close off the system and thus prevent matter from flowing in or out. The system is limited by the volume of the cylinder. In this case, the boundary of the system is not rigid, but decreases or increases with the position of the piston. If the gas is compressed, work is done on the gas by the piston – supply of work to the system. The gas then expands as a result of the combustion of the air-fuel-mixture. This is associated with a strong increase in pressure, so that the work is now done by the gas on the piston – work supplied by the system that drives the wheels.

Note: If the intake stroke or the exhaust stroke is considered, the cylinder it then an open system due to the exchange of matter across the boundary.
Adiabatic thermodynamic systems
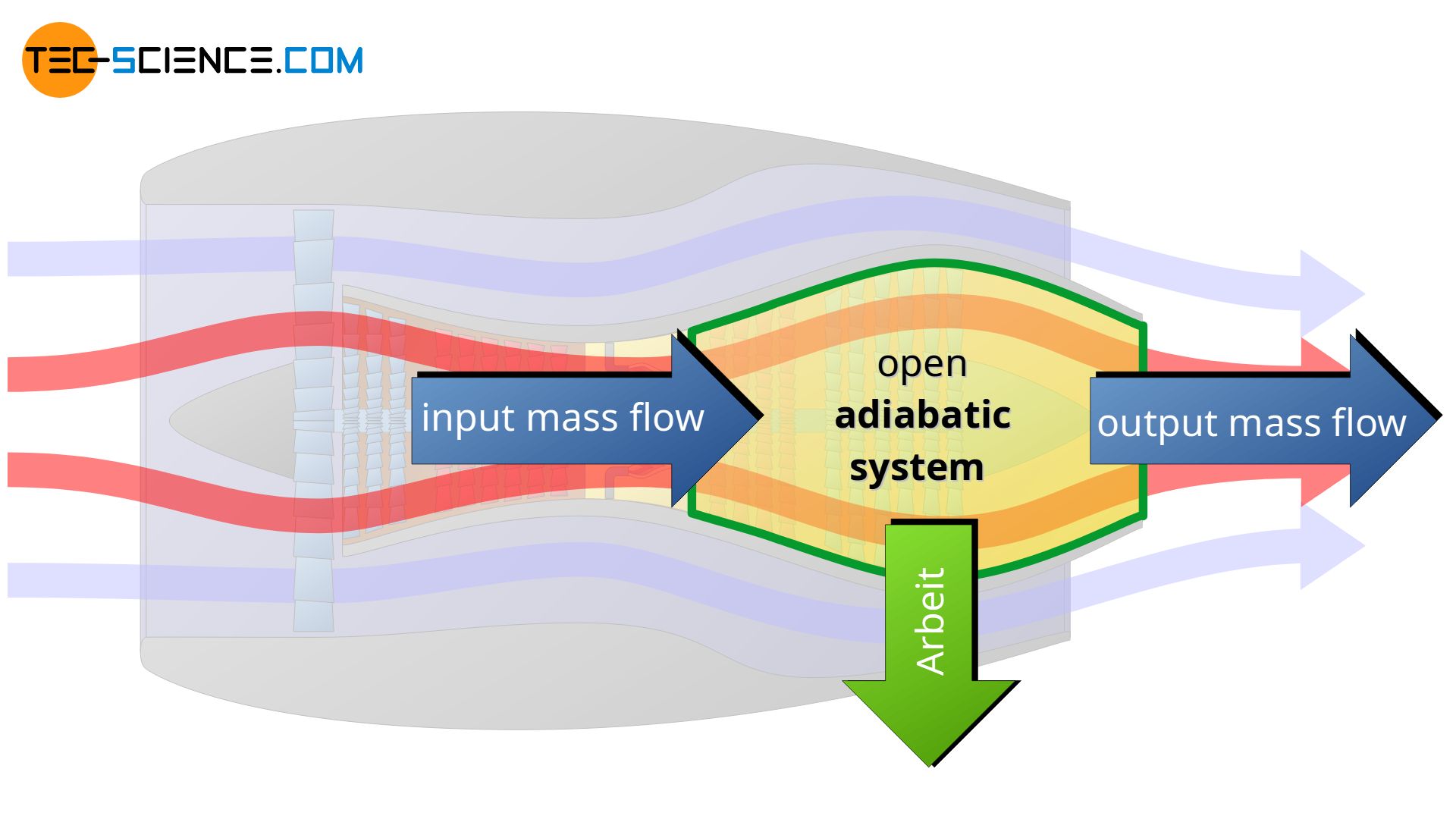
An adiabatic system can transfer energy only as work across the boundary, but no heat. An adiabatic system is therefore an idealized concept of a perfectly thermally insulated system. The adiabatic system can either be permeable to matter (open adiabatic system) or impermeable to matter (closed adiabatic system).
In reality, complete thermal insulation of a system is not possible, so adiabatic systems can only be achieved approximately. Such cases exist approximately, for example, when thermodynamic processes occur so fast that there is hardly any time for a heat exchange across the boundary. Often the compression and expansion processes in internal combustion engines or turbines are considered to take place approximately in adiabatic systems because of the short times in which they happen (high rotational speeds). So there is hardly any time left for a heat transfer in a significant way.
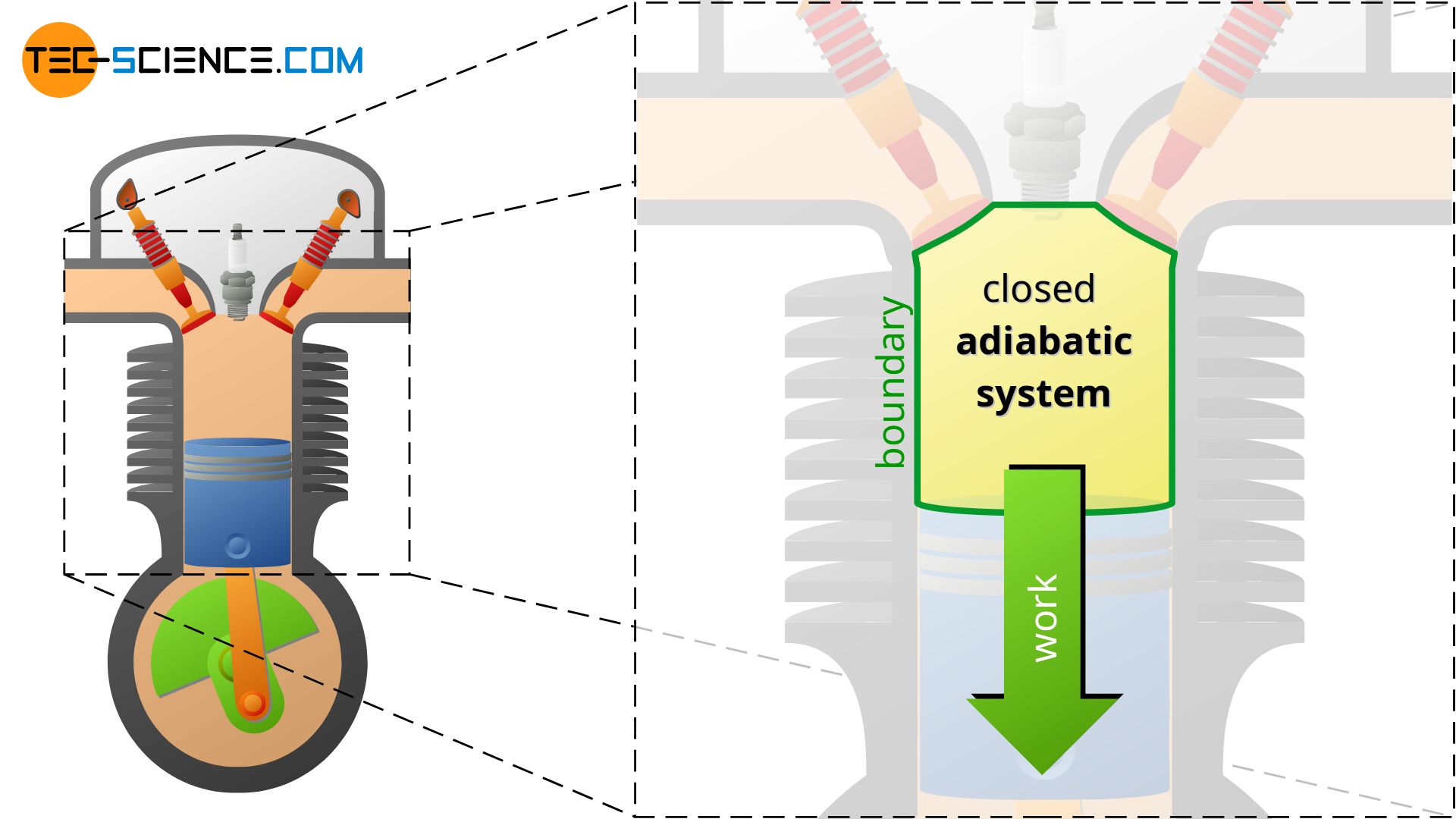
Note that in the case of the turbine shown in the figure below, the selected system boundary does not include the combustion chamber, but only the part behind it where the expansion takes place. Thus, there is no heat input for the chosen system!
Isolated thermodynamic systems
The boundary of an isolated system is neither permeable to energy (in whatever form), nor permeable to matter. Therefore, there is no interaction between the system and the surrounding. Since there is no exchange of energy of any kind, the internal energy always remains constant in an isolated system. From a thermodynamic point of view, nothing happens in such systems. An example of a closed system would be an ideal thermos flask.







