Internal energy is the sum of the different forms of energy on a microscopic level inside a substance. Learn more about it in this article.
Energy transfer as heat and as work
In the article The process quantities: Heat and work it was explained in detail that energy can be transferred to any material as (mechanical) work or as heat. The supplied energy is absorbed by the substance. This absorption of energy can then become noticeable in different ways. The different forms of energy that can benefit from such an energy transfer are discussed in the following.
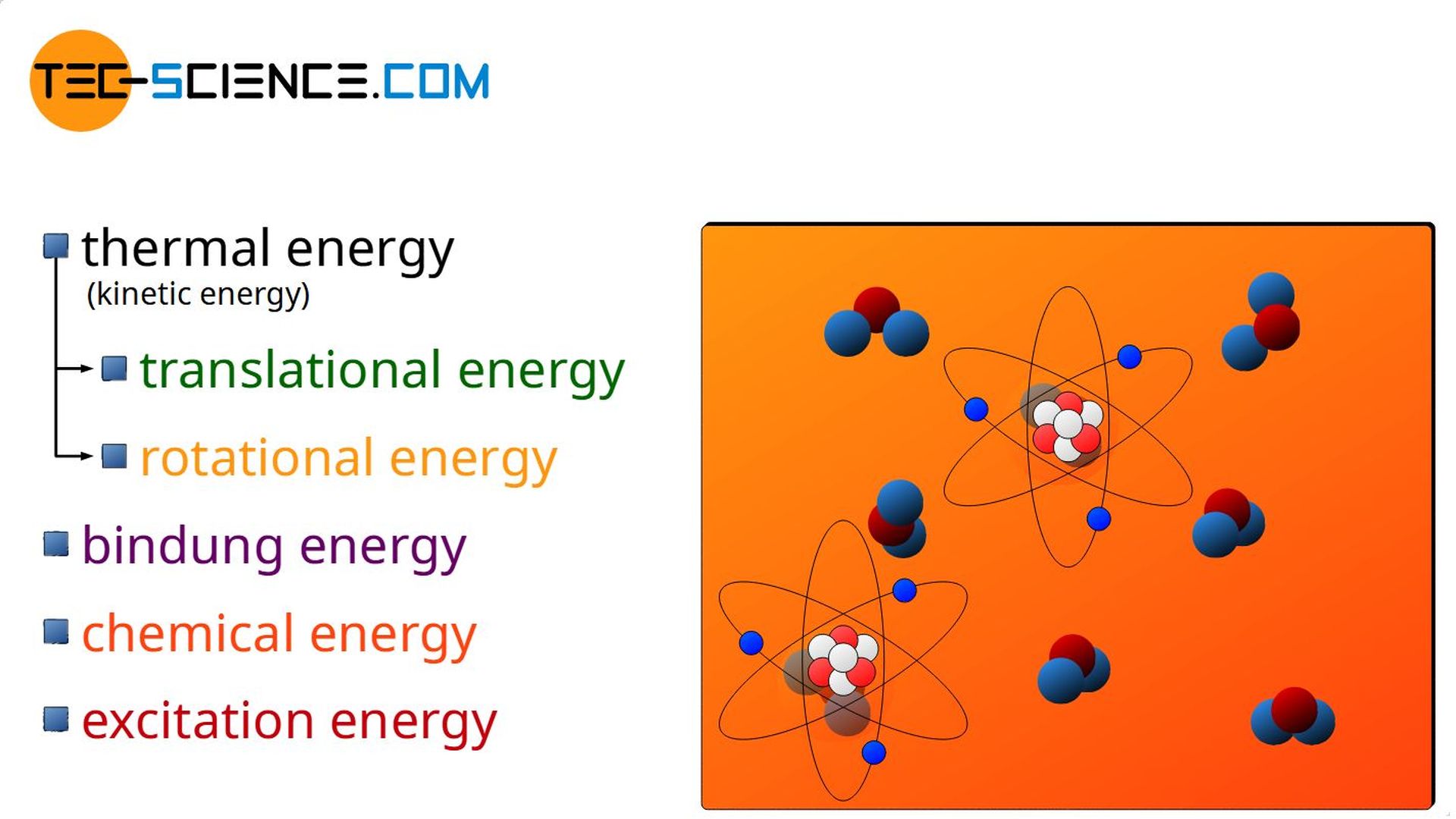
Thermal Energy
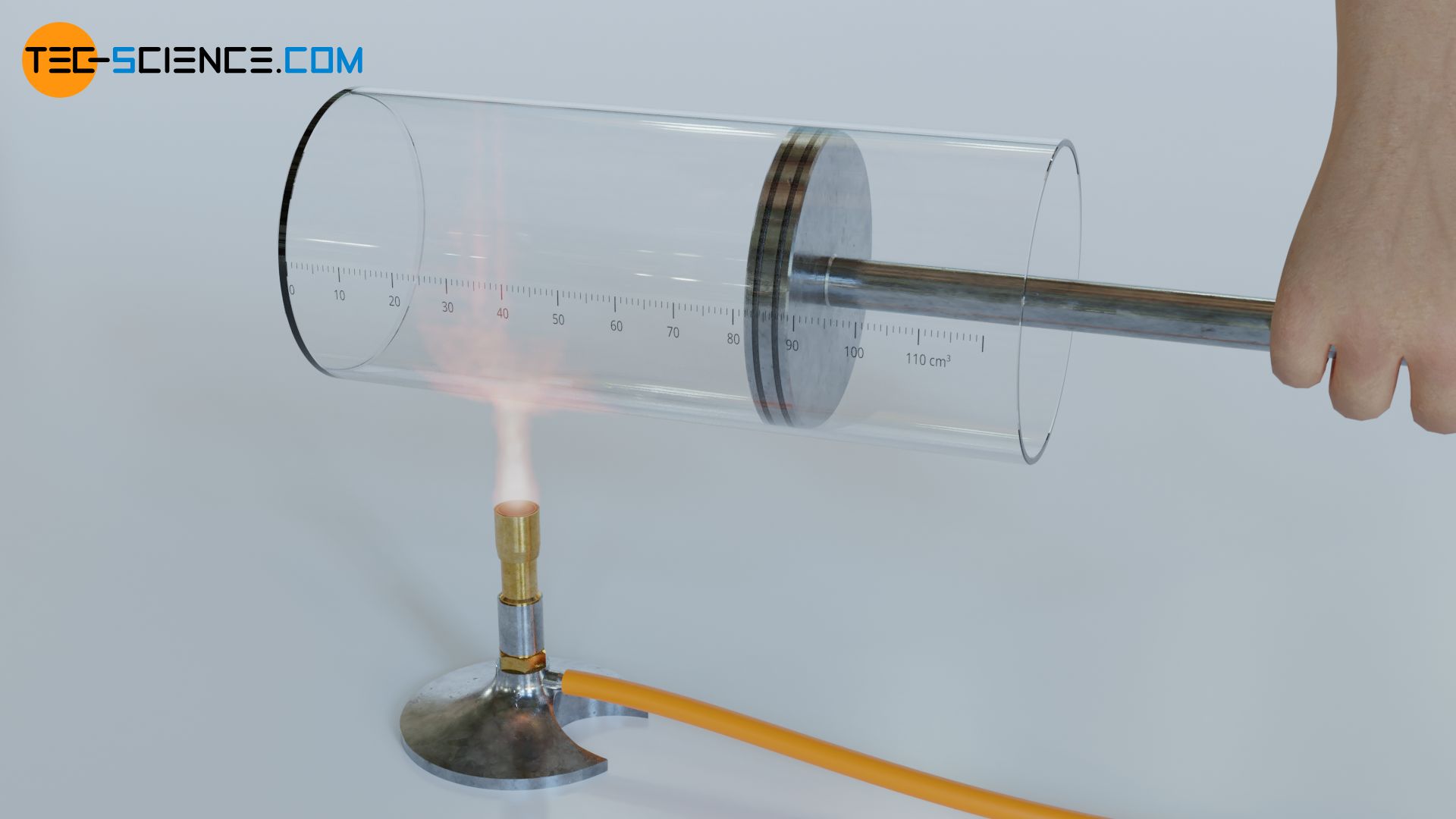
In the case of a gas, for example, a transfer of energy as heat increases the kinetic energy of the molecules. This in turn is directly related with an increase in temperature. Even if mechanical work is done by compressing the gas, the transferred energy will favor the kinetic energy of the molecules and thus increase the temperature of the gas (see also the article Why does pressure and temperature increase during the compression of a gas?).
The kinetic energy of the molecules includes both the translational motion of the centers of mass and a possible rotational motion of the molecules around their center of mass. These kinetic energies due to random molecular motions are generally referred to as thermal energy (see also article Equipartition theorem).
Thermal energy is the kinetic energy of molecuels resulting from random molecular motion (translational and rotational)!
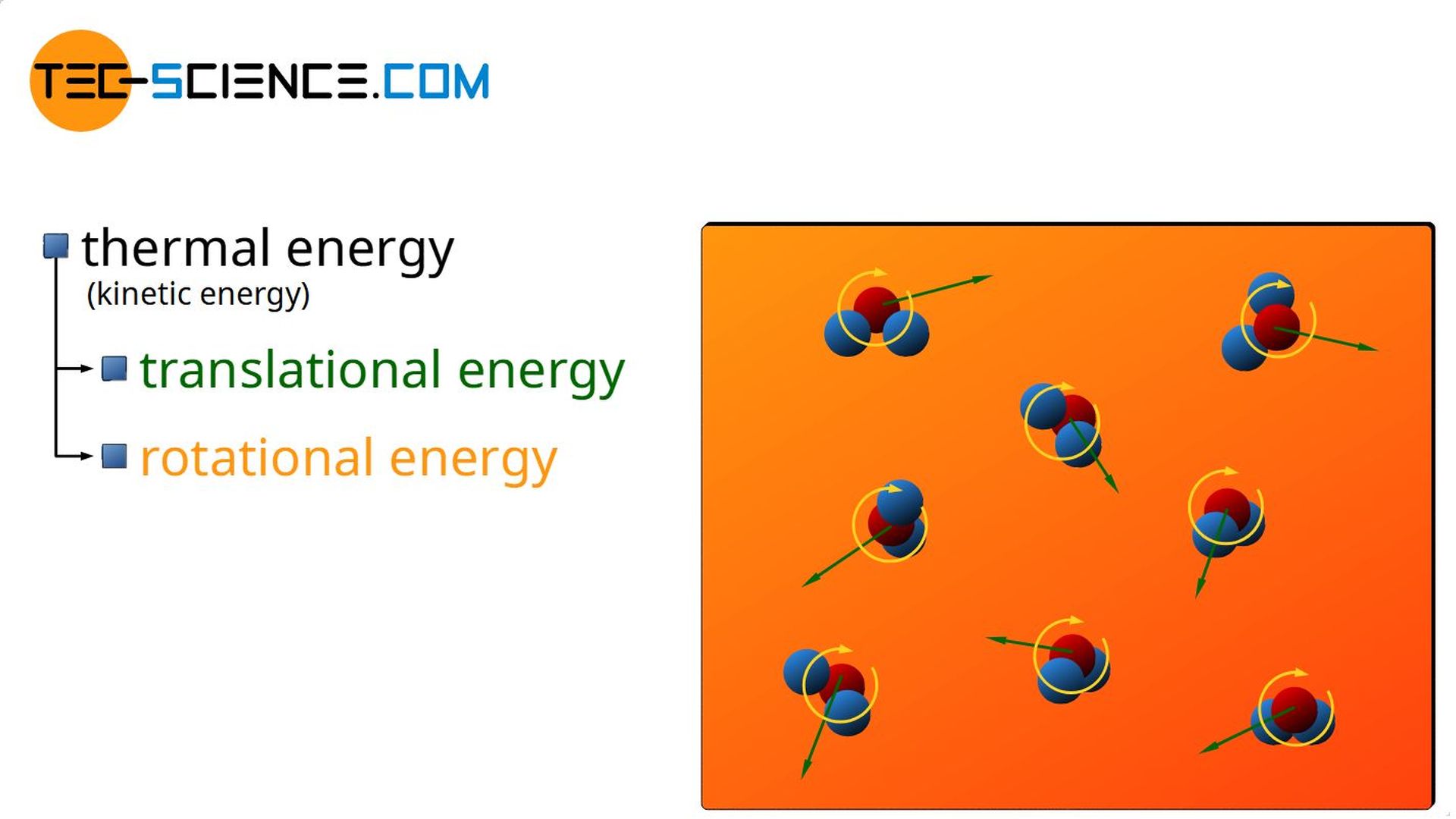
The term random or disordered in the context of molecular motion refers to the fact that, for example, water in a glass in a fast moving car does not have increased thermal energy just because the water molecules move with the increased speed of the car. The water does not have an increased temperature just because it moves at a speed! In this case, the disordered particle motion (which determines the thermal energy and thus the temperature) is only superimposed on the ordered motion of the car.
Binding energy
In gases, the transfer of work or heat mainly influences the kinetic energy of the molecules and thus the temperature. In liquids or solids, however, the supplied energies can also be noticeable in other ways.
For example, a transfer of heat can lead to a change in the binding energies. This will be the case with phase transitions. When liquids evaporate, for example, the heat transferred is not used to increase the speed of the molecules (the temperature therefore remains constant during the phase transition!), but to decrease the binding forces so that the water becomes gaseous.
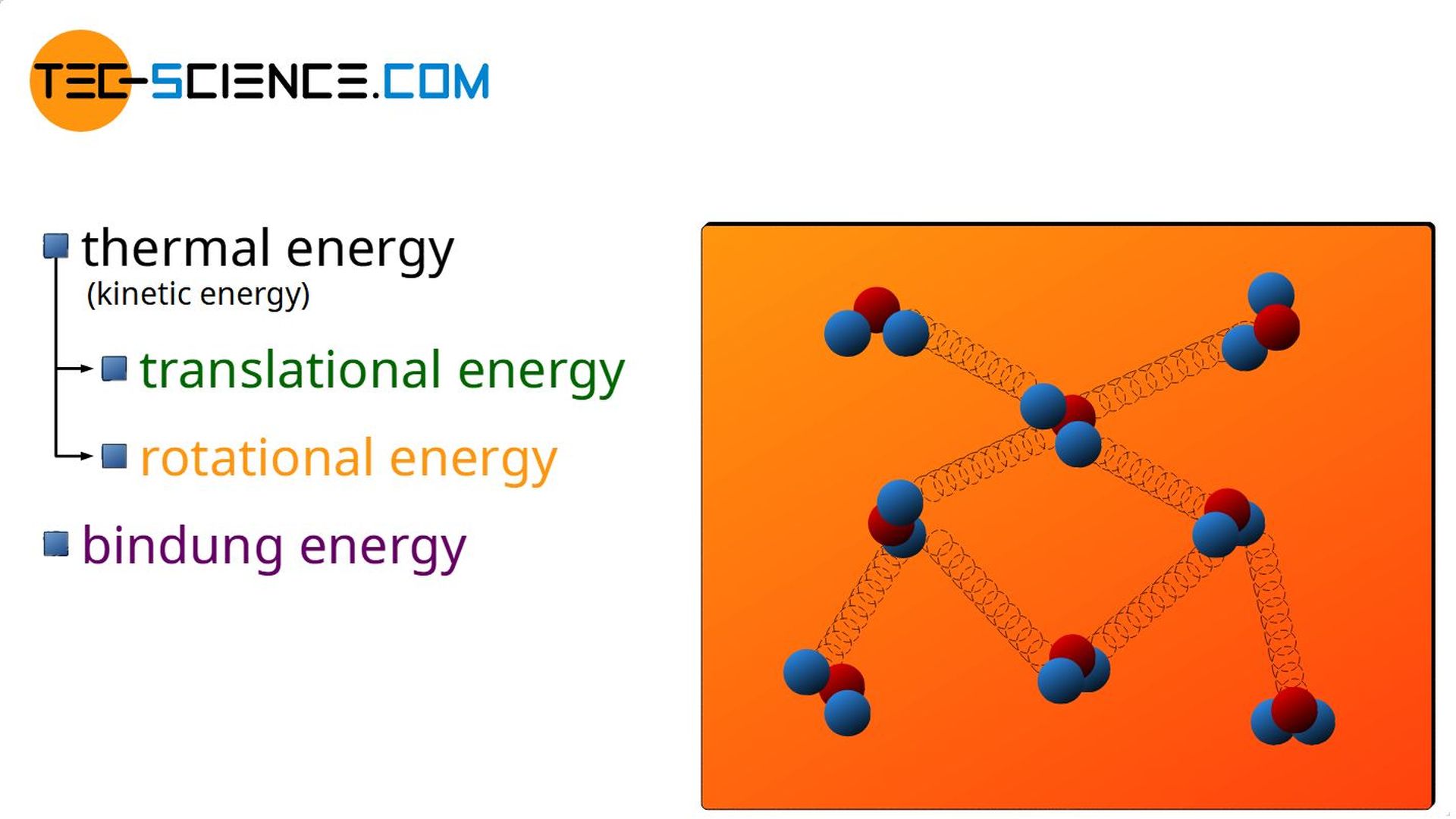
But not only energy as heat, but also a transfer of mechanical energy can have an effect on the binding energies. When a gaseous substance is compressed (work done on the gas), the gas can liquefy at high pressures. This is the case, for example, with propane gas cylinders, where the propane gas has been compressed so strongly that it is present in liquid form. Only when expanding to ambient pressure does the liquid propane gasify. Thus, work done on a gas by compression can also affect the binding energies.
Chemical energy and excitation energy
Another way in which a transfer of energy can be noticeable in substances is demonstrated by recharging a battery. In this case, the supply of electrical energy (which in thermodynamics is also regarded as work, because to produce electrical energy work has to be done by a generator!) leads to chemical reactions. The work done as electrical energy on the substances inside the battery therefore benefits the chemical energy of these substances due to chemical reactions.
Fluorescent tubes, for example, show another way how a transfer of (electrical) energy can become noticeable in a substance. The atoms of a fluorescent gas are stimulated to glow by the supplied energy. The supplied energy is therefore noticeable as excitation energy.
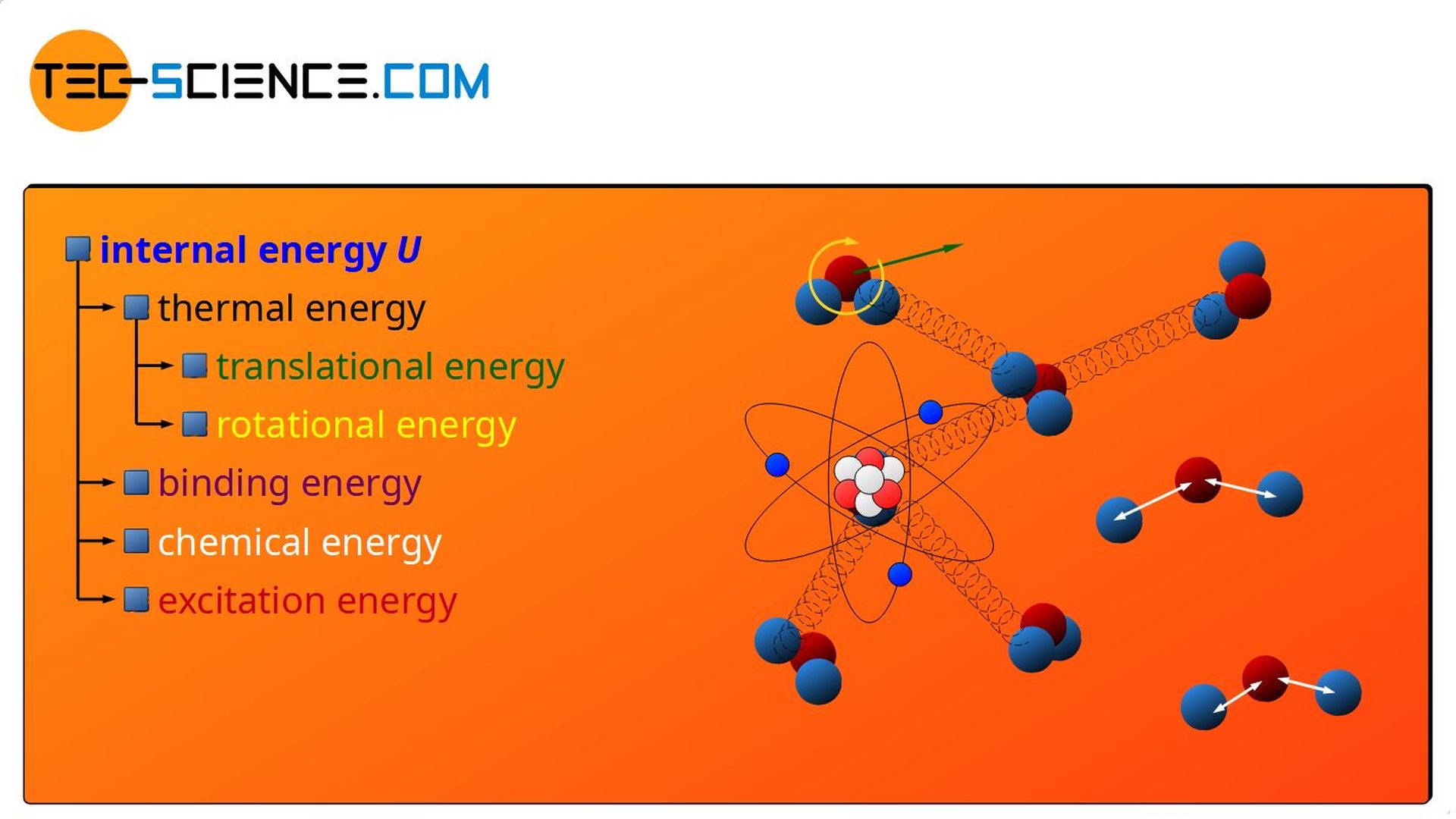
Internal Energy
The above examples show that a transfer of energy to a substance (as heat or as work) can benefit the various forms of energy present inside a substance at the microscopic level. These include the above-mentioned forms of energy such as:
- thermal energy (“disordered” kinetic energy in the form of translation and rotation),
- binding energy,
- chemical energy and
- excitation energy
There are certainly even more forms of energy inside a substance that could benefit from a supply of energy. So this list is not complete! But all these forms of energy, which in principle can be present inside a substance at a microscopic level, are summarized under the term internal energy!
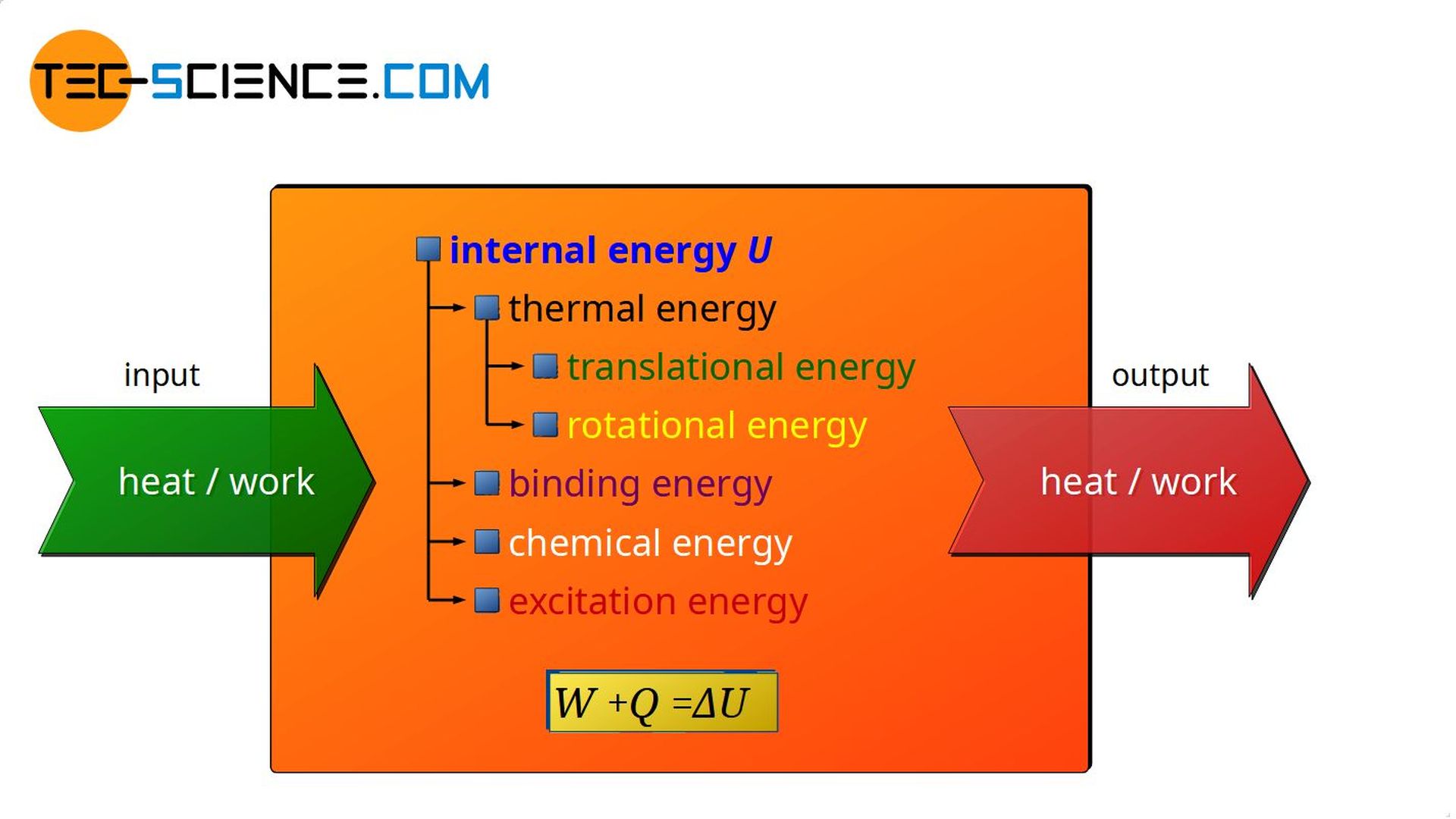
Therefore, the general rule is: If energy is transferred to a substance as heat or as work, this always favors the internal energy. The internal energy of the substance will increase in this case. Which forms of energy are exactly affected by such an increase of internal energy (whether thermal energy, binding energy, chemical energy, excitation energy or a combination of these) depends on the substance and the exact boundary conditions.
Conversely, if a substance releases energy as heat or by doing work, this is always at the expense of its internal energy. Whether thermal energy, binding energies, chemical energies or excitation energies are affected depends again on the substance and the exact boundary conditions.
First law of thermodynamics
Heat Q and work W transferred to a substance (into a thermodynamic system) or transferred by a substance (out of a thermodynamic system) do not just disappear or appear out of nowhere, but rather lead to a change in the internal energy ΔU of the substance (thermodynamic system). This statement of the law of conservation of energy is also called the first law of thermodynamics. Mathematically, the first law of thermodynamics can be expressed as follows:
\begin{align}\;\;\;\;\;
&\boxed{ Q+W=\Delta U } \\[5px]
\end{align}
In contrast to the process quantities heat and work, which describe the process of the transfer of energy (“energy in transit”), the internal energy is a state quantity, which describes the energetic state of the substance at a molecular level (“energy in possession”)!
If the internal energy of a substance increases, then the change in internal energy is positive (ΔU>0). Conversely, the change in internal energy is counted negative (ΔU<0) when the internal energy decreases. This results in the following sign convention for a transfer of heat or work:
If heat or work is transferred into a thermodynamic system, these amounts of energy are counted positively (Q> or W>0). If heat or work is transferred out of a thermodynamic system, these amounts of energy are counted negatively (Q<0 or W<0)!
Note the terminology for gases:
- work done on the gas: energy transfer from the surroundings to the gas by compression -> W>0
- work done by the gas: energy transfer from the gas to the surroundings by expansion -> W<0
- heating of the gas: energy transfer from the surroundings to the gas -> Q>0
- cooling of the gas: energy transfer from the gas to the surroundings -> Q<0
Change of internal energy
The first law of thermodynamics only makes a statement about the change in internal energy of a substance! The exact amount of internal energy U cannot be determined in practice due to the many forms of energy that could be present and the enormous number of molecules in a substance. Only for ideal gases, such a determination of the internal energy is possible.
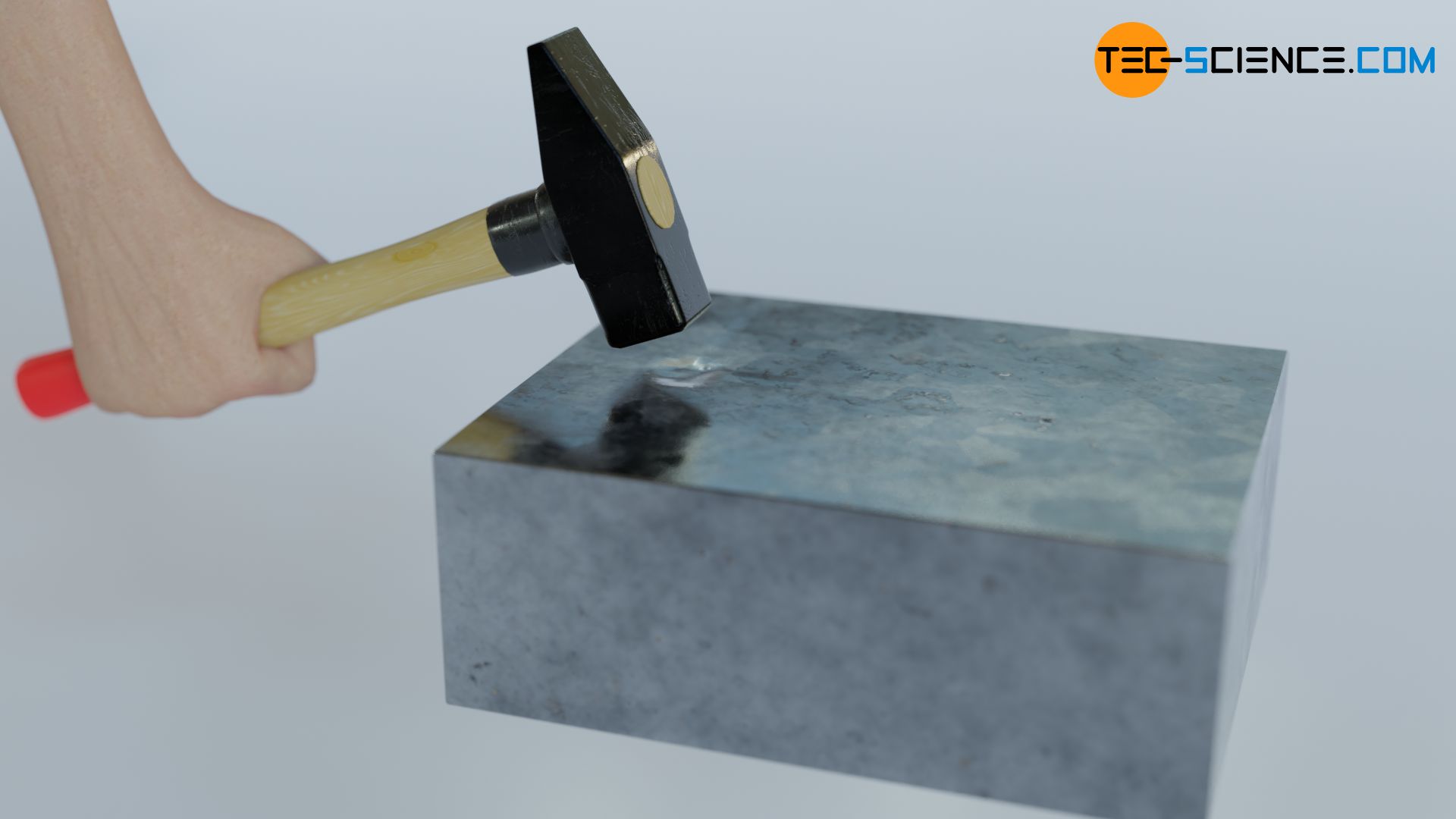
It is now also clear that a substance basically cannot contain heat in the thermodynamic sense of the word, but can only have internal energy (in whatever exact form). Whether the internal energy was changed by a transfer of work or by a transfer of heat, cannot be determined afterwards anyway. The following thought experiment should illustrate this.
Imagine a solid body at absolute zero. The molecules contained in the substance therefore have no kinetic energy whatsoever. In thought, the structure of the body is now set in motion by a hammer (transfer of energy as work). The solid body thus reaches its temperature in a purely mechanical way, without ever having been heated. Therefore, heat (or work) can never be contained in a substance, but only internal energy!
In thermodynamics heat refers only to the transfer of energy (“energy in transit”) from an object with a higher temperature to an object with a lower temperature due to collisions between molecules or due to radiation (see also the article Heat and thermodynamic equilibrium).






