Vapor pressure thermometers use the temperature-dependent vapor pressure of a liquid as the measuring principle.
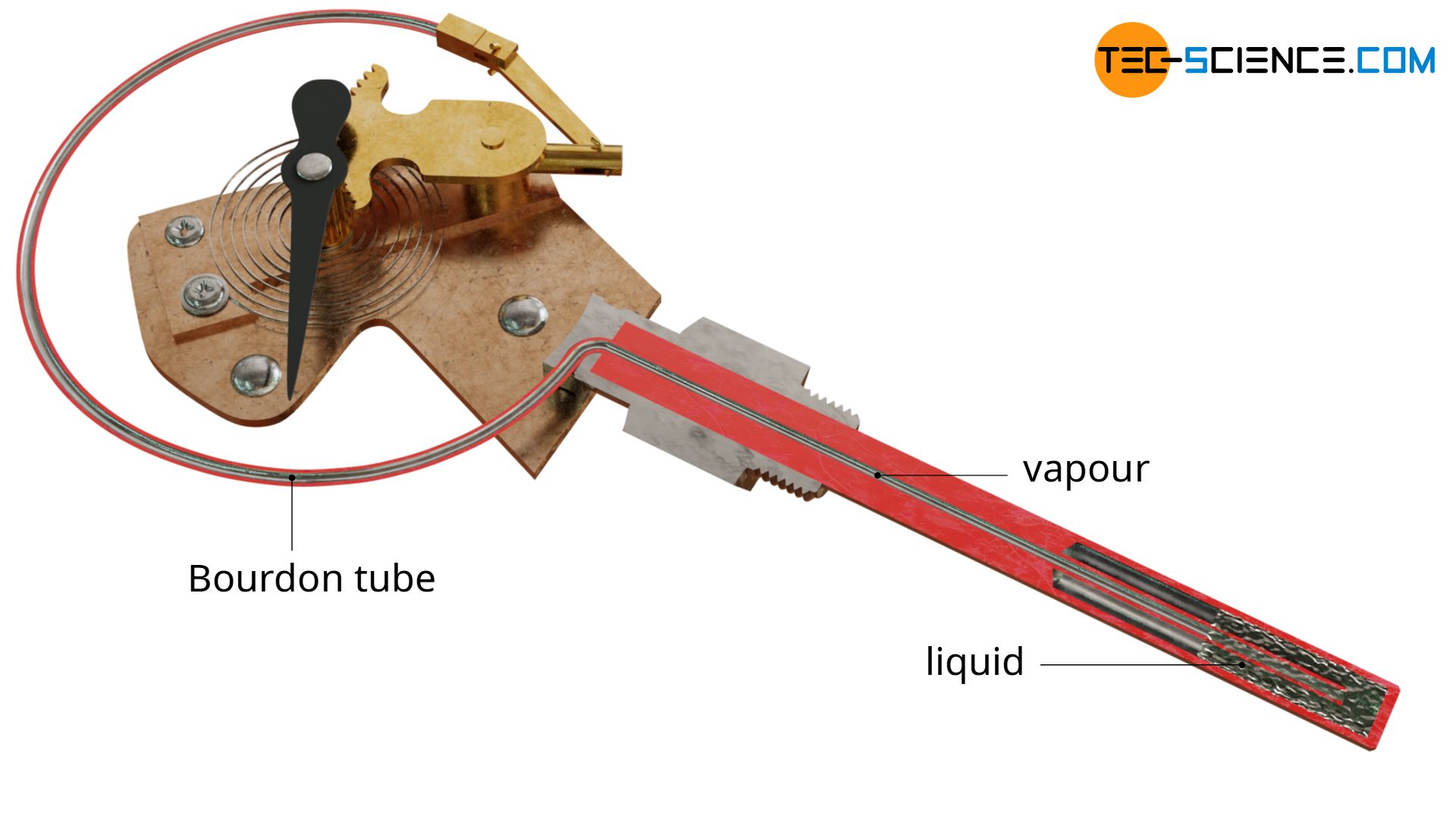
The less popular vapour pressure thermometers work according to the same principle as liquid-in-metal thermometers or gas-in-metal thermometers. For this reason, they are also referred to as vapour-in-metal thermometers.

In vapour pressure thermometers a highly volatile liquid is used, but only partially occupies the thermometer volume. The liquid begins to vaporize and fills the rest of the volume with steam. As a result, the pressure rises until an equilibrium between the liquid phase and the gas phase is reached. In simple terms, the rising pressure causes the vaporizing particles to be forced back into the liquid state.
This equilibrium between vapour and liquid depends on the temperature (cf. vapour pressure curve). If the temperature rises, more liquid will vaporize and the vapour pressure will rise accordingly (see also the article Evaporation of liquids). This rising vapour pressure is indicated by a Bourdon tube, as with gas filled thermometers. Conversely, a drop in temperature causes part of the gaseous vapour to condense and the vapour pressure to decrease. The indicated temperature decreases.
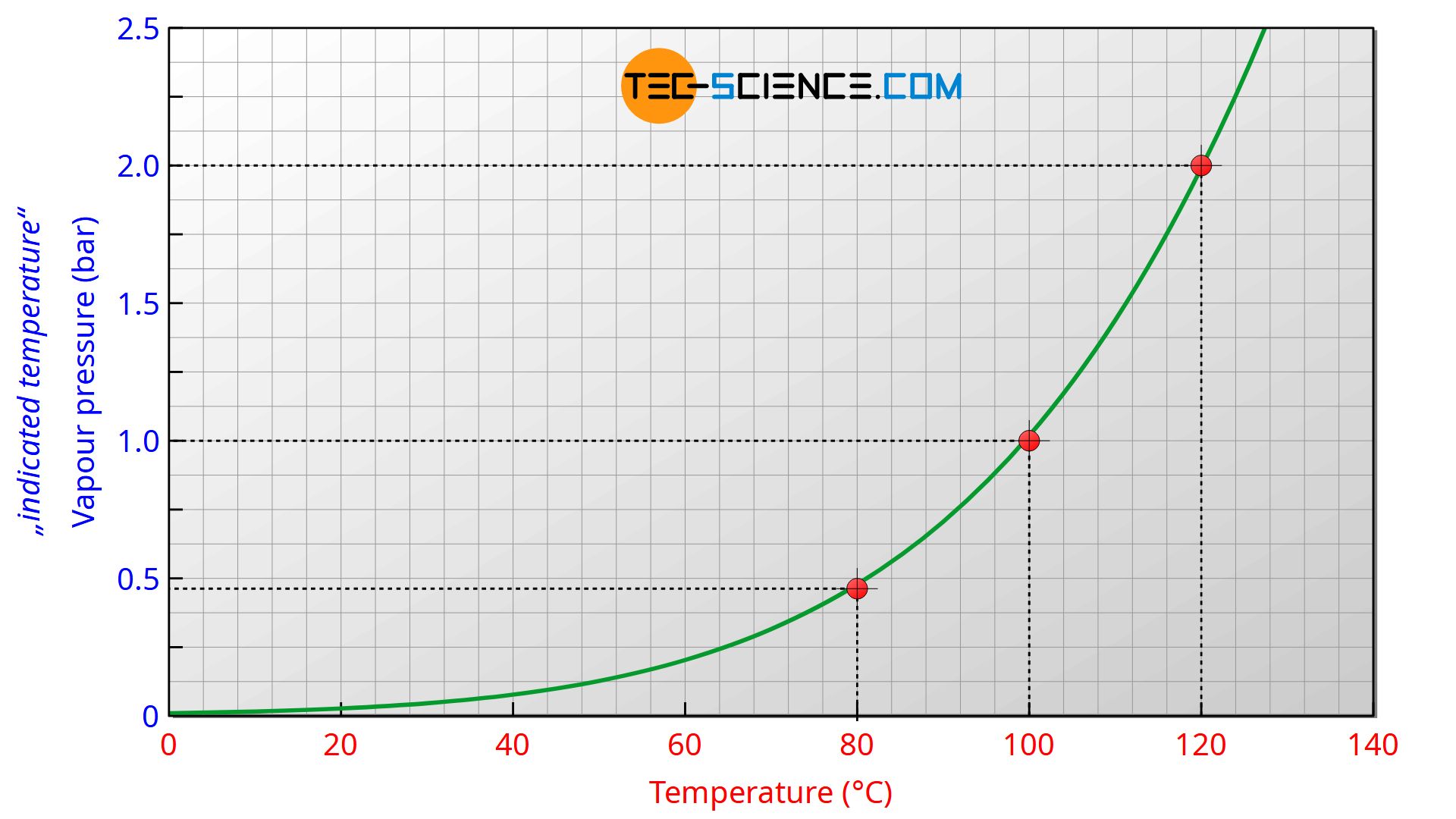
Since the vapour pressure curves are not linear, the scale for vapour pressure thermometers is not evenly subdivided. Due to the exponentially increasing vapour pressure curves, the scale distances increase with higher temperature, so that the measurement sensitivity and accuracy increases as a result. Vapour pressure thermometers should therefore be used in the upper third of the scale for sufficient measurement accuracy.

The figure below shows the vapour pressure curve of water as an example.
Vapour pressure thermometers can be used at very low temperatures, which are only a few Kelvin above absolute zero.