The specific latent heat of vaporization (enthalpy of vaporization) is the amount of heat required to vaporize a liquid substance!
Process of vaporization
If a liquid is heated more and more, then at some point the boiling point is reached. At this point, the state of matter changes and the liquid finally begins to vaporize (also referred to as boiling). During vaporization, no further increase in temperature is observed for pure substances, despite the continued supply of heat energy. During vaporization, the energy obviously no longer benefits the increase of the kinetic energy of the molecules, which would otherwise mean an increase in temperature (see also article Temperature and particle motion).
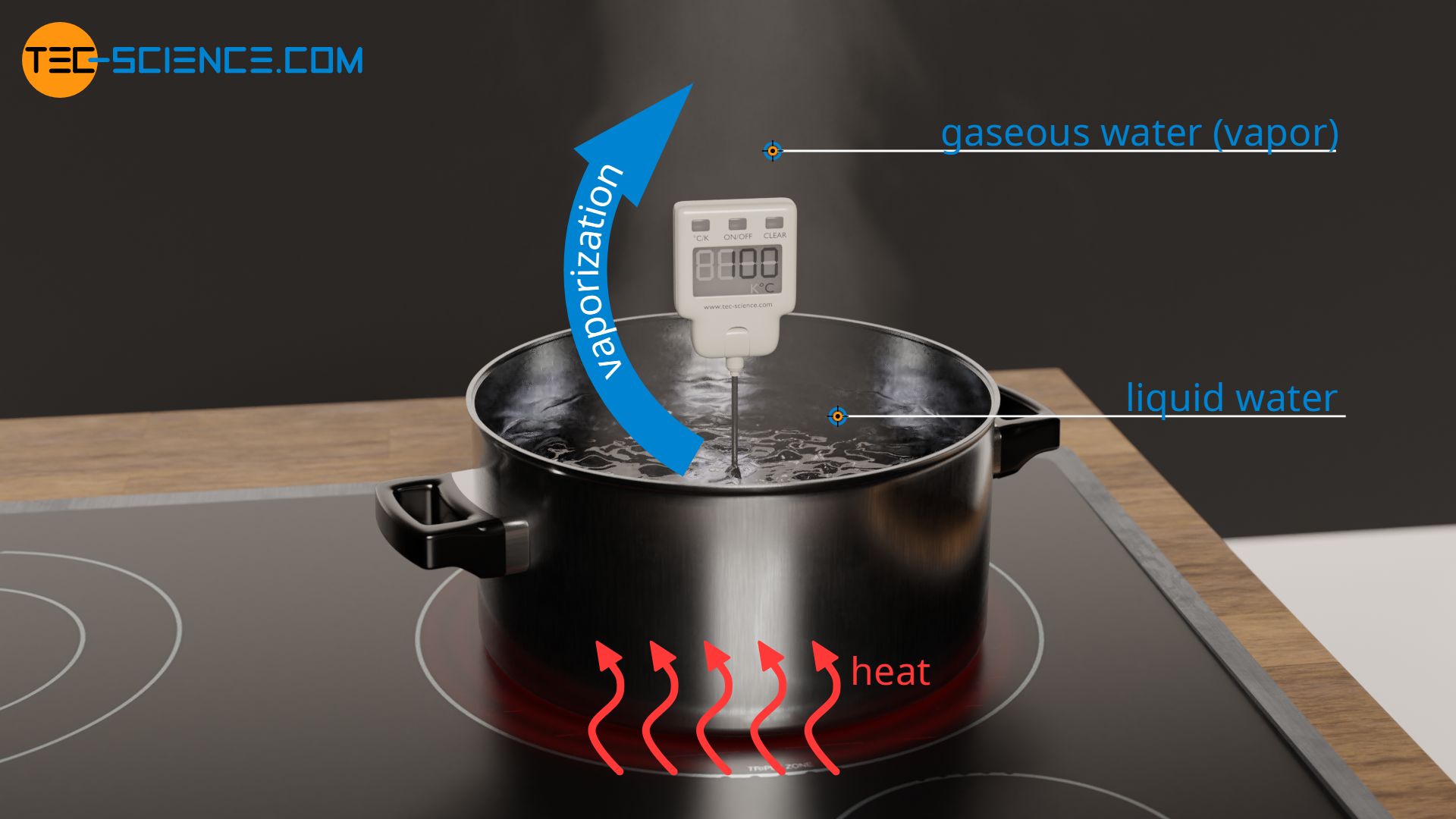
During vaporization, the transferred energy leads to an increase in the internal energy in terms of changed binding energies between the molecules in the liquid and gaseous state. The intermolecular bonds in the liquid state are being broken by the added heat energy, thus allowing the transition to the gaseous state. In the gaseous state, the molecules are only relatively weakly bonded to each other due to the lower binding forces.
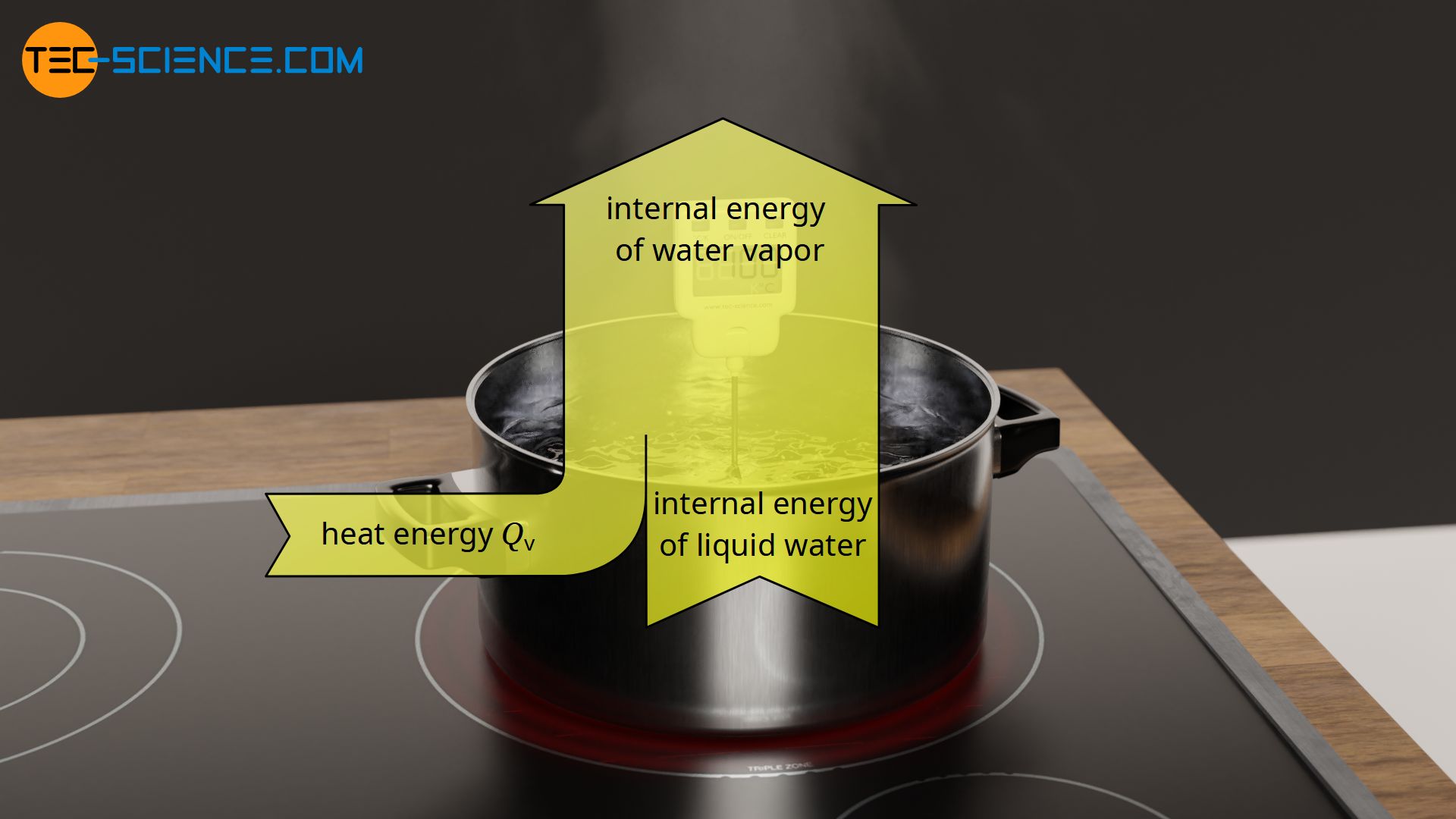
During vaporization, energy must be added to break the intermolecular bonds. In the case of pure substances, the temperature of the liquid remains constant until the process of vaporization is completed!
More detailed information on this can also be found in the article Why does the temperature remain constant during a change of state (phase transition)?
The fact that heat has to be supplied permanently to drive the breaking of intermolecular bonds is evident, for example, in the boiling of water. If water is boiled in a pot, the water only vaporizes as long as the hotplate remains switched on. However, if the heat supply is interrupted, the water also stops boiling.
The question arises as to how much heat must be added in order to completely vaporize a certain amount of a liquid. The heat required for this is also referred to as the heat of vaporization or enthalpy of vaporization. This heat of vaporization does not include the amount of heat required to heat the liquid to the boiling point. The heat of vaporization therefore only includes the heat energy to be added during vaporization if the liquid has already been heated to boiling temperature.
The heat of vaporization (enthalpy of vaporization) is the heat energy to be added to a liquid at its boiling point in order to completely vaporize a certain amount of the substance!
For the difference between the terms heat and enthalpy, see the article Difference between latent heat of vaporization and enthalpy of vaporization.
Since the heat of vaporization added during vaporization is not directly noticeable in an increase in temperature, but can nevertheless be found in the vaporized substance in the form of internal energy, the heat of vaporization is also referred to as latent heat. The term “latent” comes from Latin and means “to be hidden” or “not to appear directly”.
Experimental determination of the heat of vaporization
Experimental setup
Using water as an example, the heat of vaporization required to vaporize a certain amount of water is to be determined experimentally in the following. For this purpose, water is first heated to boiling temperature with an immersion heater. Then, the vaporization of the water mass over time is observed using a balance on which the experimental setup is placed.
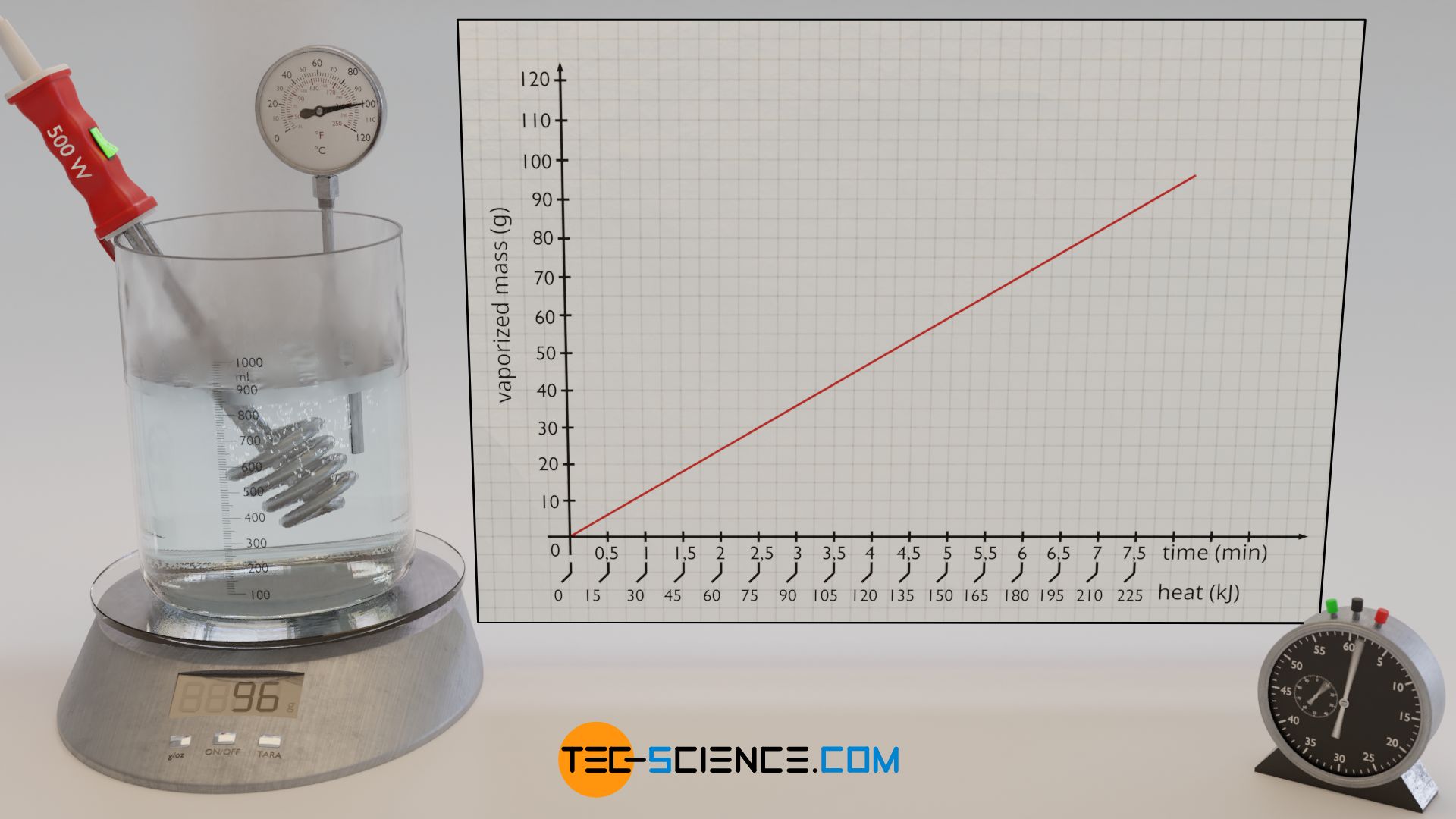
The added heat of vaporization can be determined via the electrical power of the immersion heater, which is completely converted into heat. The heat energy Qv (= heat of vaporization) added at a power P is obtained by the operating time t of the immersion heater according to the following formula:
\begin{align}
\label{q}
Q_\text{v} = P \cdot t \\[5px]
\end{align}
Observation
First, the water is heated to boiling temperature with the immersion heater. When the water begins to vaporize, the experiment can be started at any time. To do this, the balance is reset to zero and the time measurement is started. The water gradually vaporizes and the vaporized mass is displayed on the balance. At regular time intervals, the displayed mass of the balance is recorded. At each time t, the added heat of vaporization Qv up to that point can be determined using formula (\ref{q}). In this way one obtains a statement which amount of heat led to the vaporization of which mass mv.
Evaluation
If one plots the vaporized mass as a function of the added heat, then a proportional relationship becomes apparent. This means that in order to vaporize twice the amount of water, twice the amount of heat must be added. The evaluation of the experimental data shows that about 120 kJ of heat are necessary to vaporize 48 g of water. With a supplied heat energy of about 240 KJ, twice the water mass of 96 g has then finally vaporized.
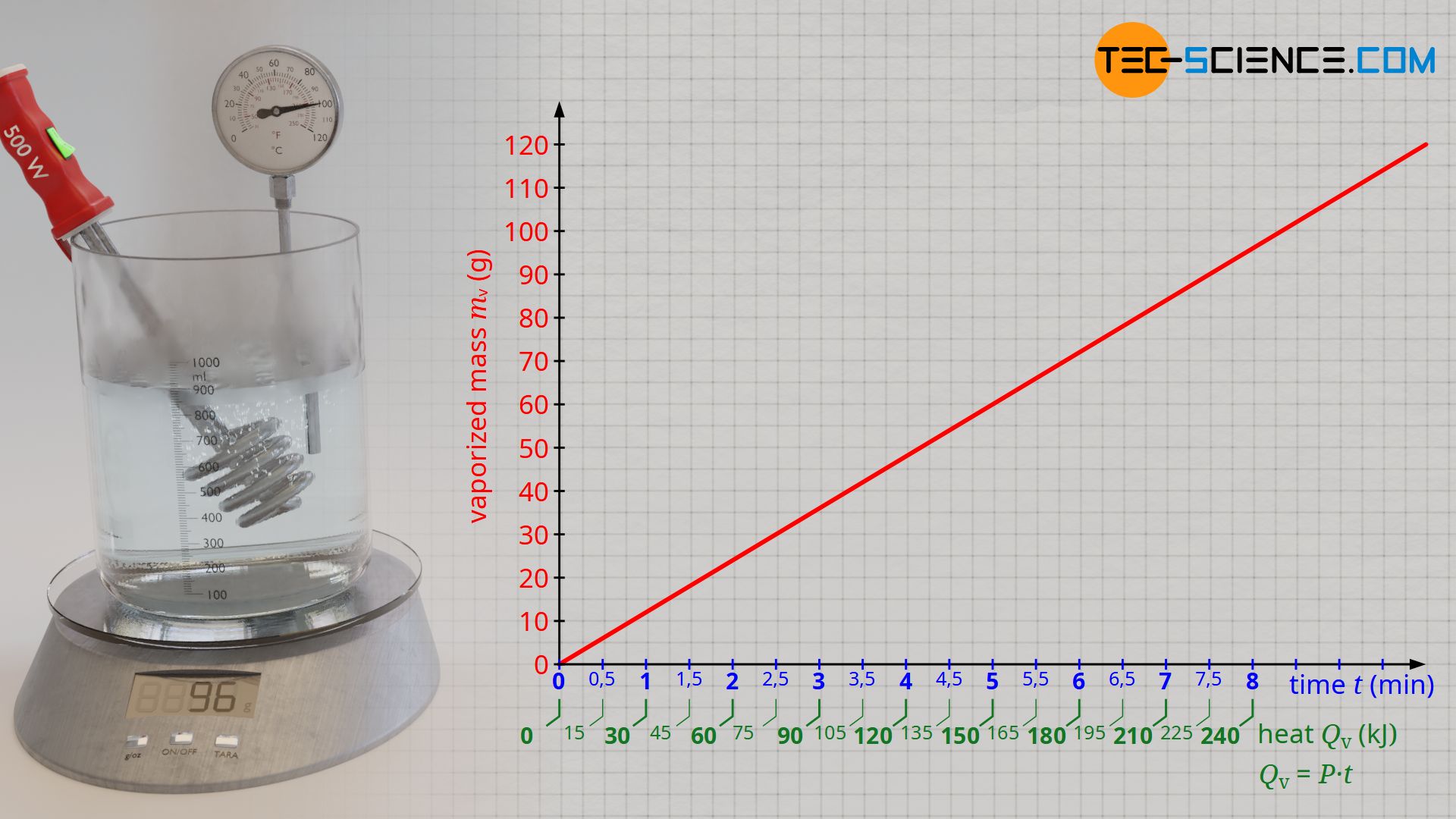
Especially with regard to the comparability of the heats of vaporization of different liquids, it therefore makes sense to always relate the heats of vaporization Qv to a standardized amount of mass to be vaporized (e.g. 1 kilogram or 1 gram). This constant ratio between the heat of vaporization and the mass mv to be vaporized is called specific heat of vaporization or specific enthalpy of vaporization qv:
\begin{align}
&\boxed{q_\text{v} = \frac{Q_\text{v}}{m_\text{v}}}~~~[q_\text{v}]=\frac{\text{J}}{\text{kg}}~~~~~\text{specific heat of vaporization} \\[5px]
\end{align}
From the experiment, a specific heat of vaporization of around 2500 kJ/kg is finally obtained for water. This means that 2500 kJ of heat is required to vaporize 1 kilogram of water. However, with the experimentally determined heat of vaporization using the described experimental setup, it must be noted that the heat emitted by the immersion heater does not completely benefit the vaporization of the water. Some of the heat is also used to heat the vessel and is thus transferred to the surroundings. Therefore, a lower amount of heat is used for the vaporization of the water than calculated with formula (\ref{q}). The literature value for the specific heat of vaporization of water is therefore somewhat lower with 2257 kJ/kg.
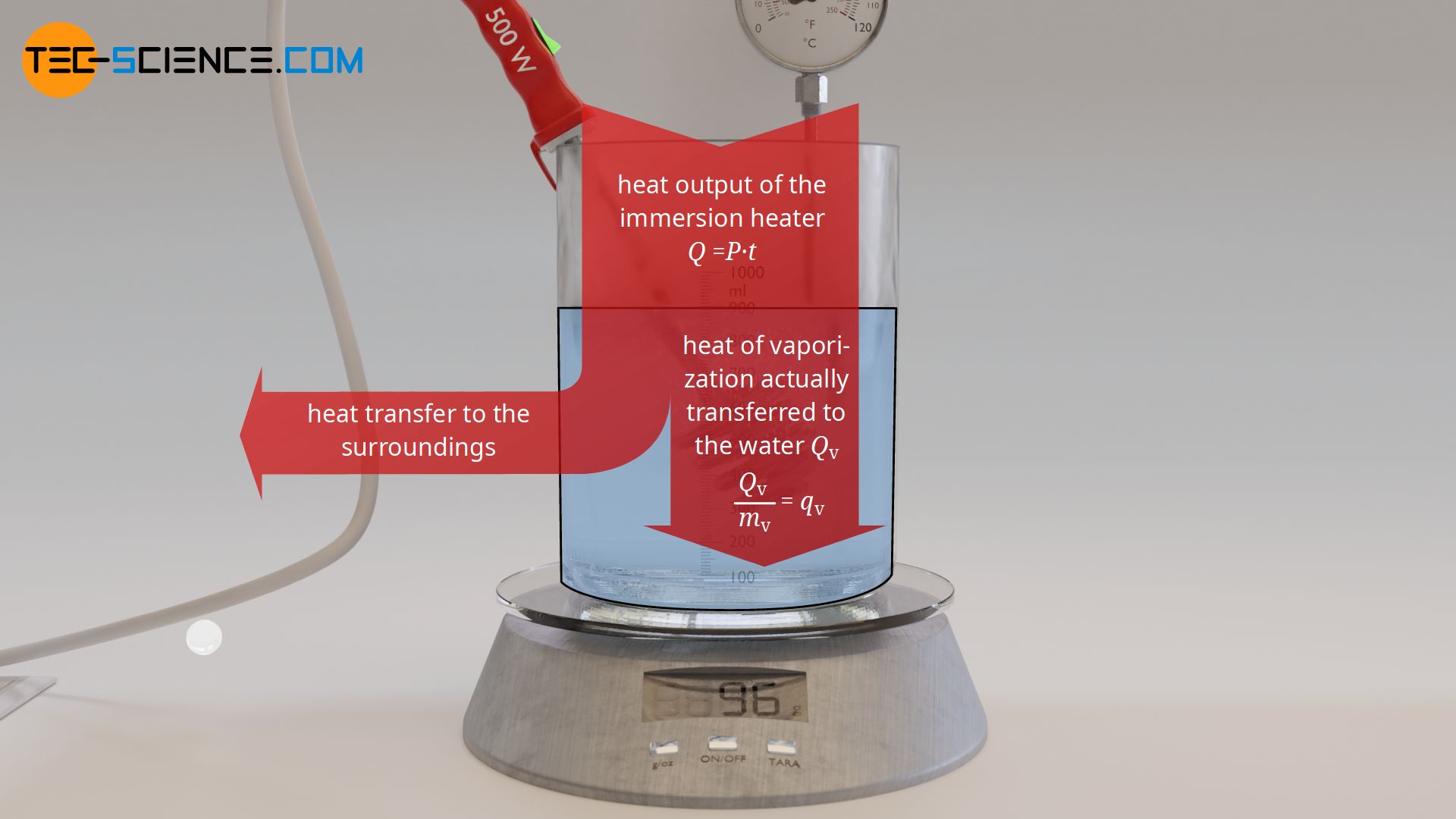
Specific heat of vaporization is the heat of vaporization to be added per unit mass of a liquid to be vaporized!
Conclusion
The specific heat of vaporization qv describes the relationship between the mass to be vaporized mv and the heat of vaporization to be added for this purpose Qv:
\begin{align}
&\boxed{Q_\text{v} = q_\text{v} \cdot m_\text{v}} ~~~\text{heat of vaporization} \\[5px]
\end{align}
In the case of water, the heat of vaporization to be added is more than five times as great as the amount of heat that would have had to be used to heat the water from 0 °C to 100 °C. This relatively large heat of vaporization is one of the reasons why a fire is extinguished excellently with water.
Specific heat of vaporization of selected substances
If the experiment described above is carried out with a water-alcohol mixture or with other liquids instead of pure water (e.g. molten metals that are vaporized), it can be seen that these substances vaporize at different rates. Consequently, more or less heat energy is required to vaporize a given mass of the substance. The specific heat of vaporization is therefore dependent on the substance.
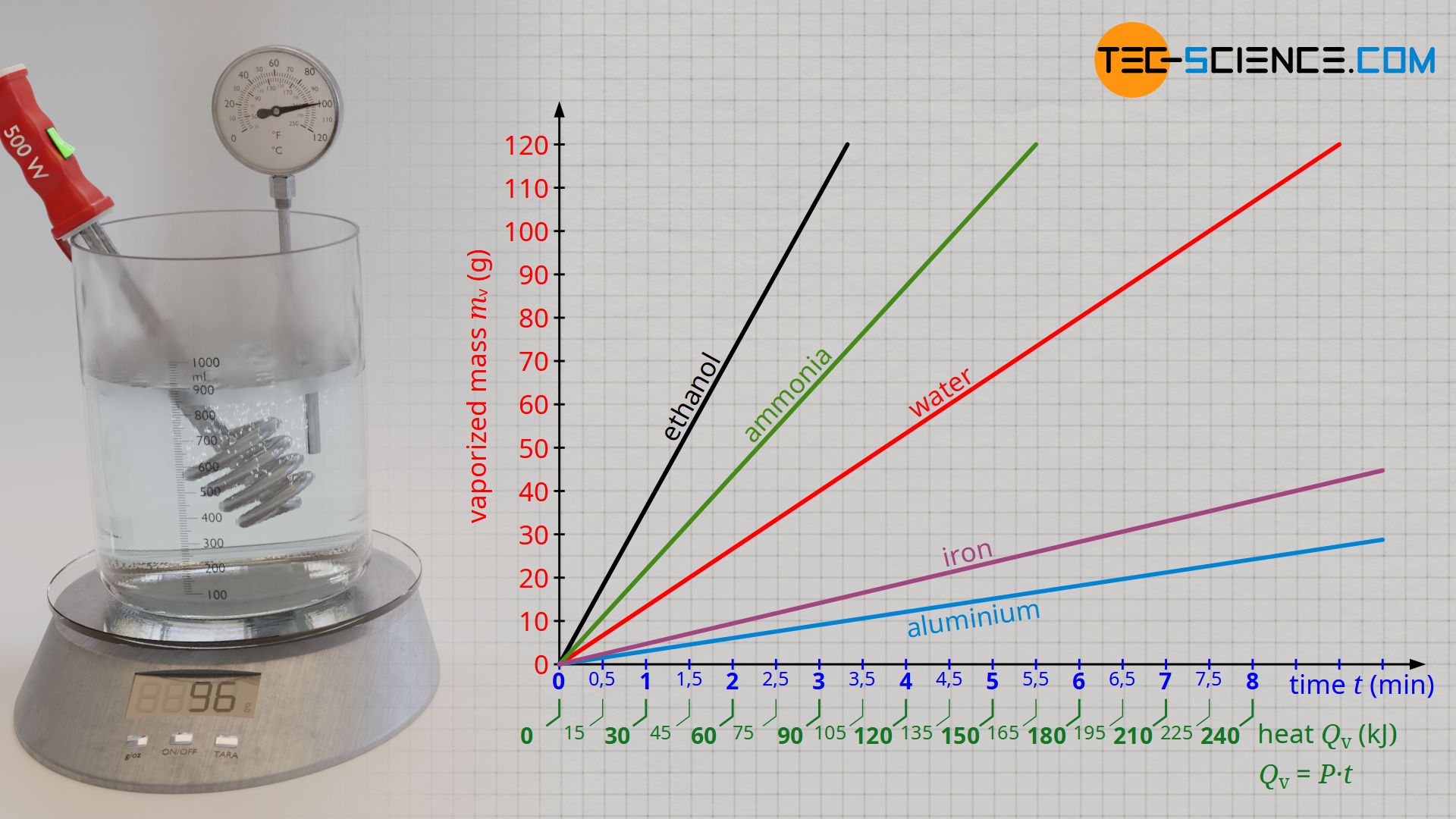
The greater the specific heat of vaporization of a substance, the more heat is required to vaporize a given mass. Substances with large specific heats of vaporization therefore do not vaporize as quickly. The table below shows the specific heats of vaporization of selected liquids.
It should be noted that the specific heats of vaporization are indirectly influenced by the ambient pressure, since this changes the boiling temperatures (see also the article Why does water boil faster at high altitudes?)! However, since most liquids are vaporized at an ambient pressure of 1 bar, the specific heat of vaporization usually refers to the boiling temperature at 1 bar.
| Substance | Boiling temperature in the unit °C at 1 bar | Specific heat of vaporization in the unit kJ/kg |
|---|---|---|
| Feststoffe | ||
| Aluminium | 2450 | 10500 |
| Lead | 1750 | 870 |
| Iron | 2865 | 6300 |
| Liquids | ||
| Ethanol | 78 | 845 |
| Mercury | 357 | 290 |
| Water | 100 | 2257 |
| Gases | ||
| Ammonia | -33 | 1370 |
| Butane | -1 | 380 |
| Propane | -42 | 430 |
Note that the temperature of not all liquids remains constant during vaporization! For example, petroleum as a mixture of different substances does not vaporization at a boiling point, but within a boiling range. In the case of petroleum, this boiling range is between 180 °C and 330 °C. The heat added during the phase transition is therefore used both to raise the temperature and to drive the vaporization. It is therefore not possible to determine exactly what proportion of the heat added is used for the temperature increase or for the vaporization. Consequently, no (specific) heat of vaporization can be assigned to such a mixture of substances. In general, such a boiling range occurs with mixtures of substances, whereas pure substances usually have a boiling point.






