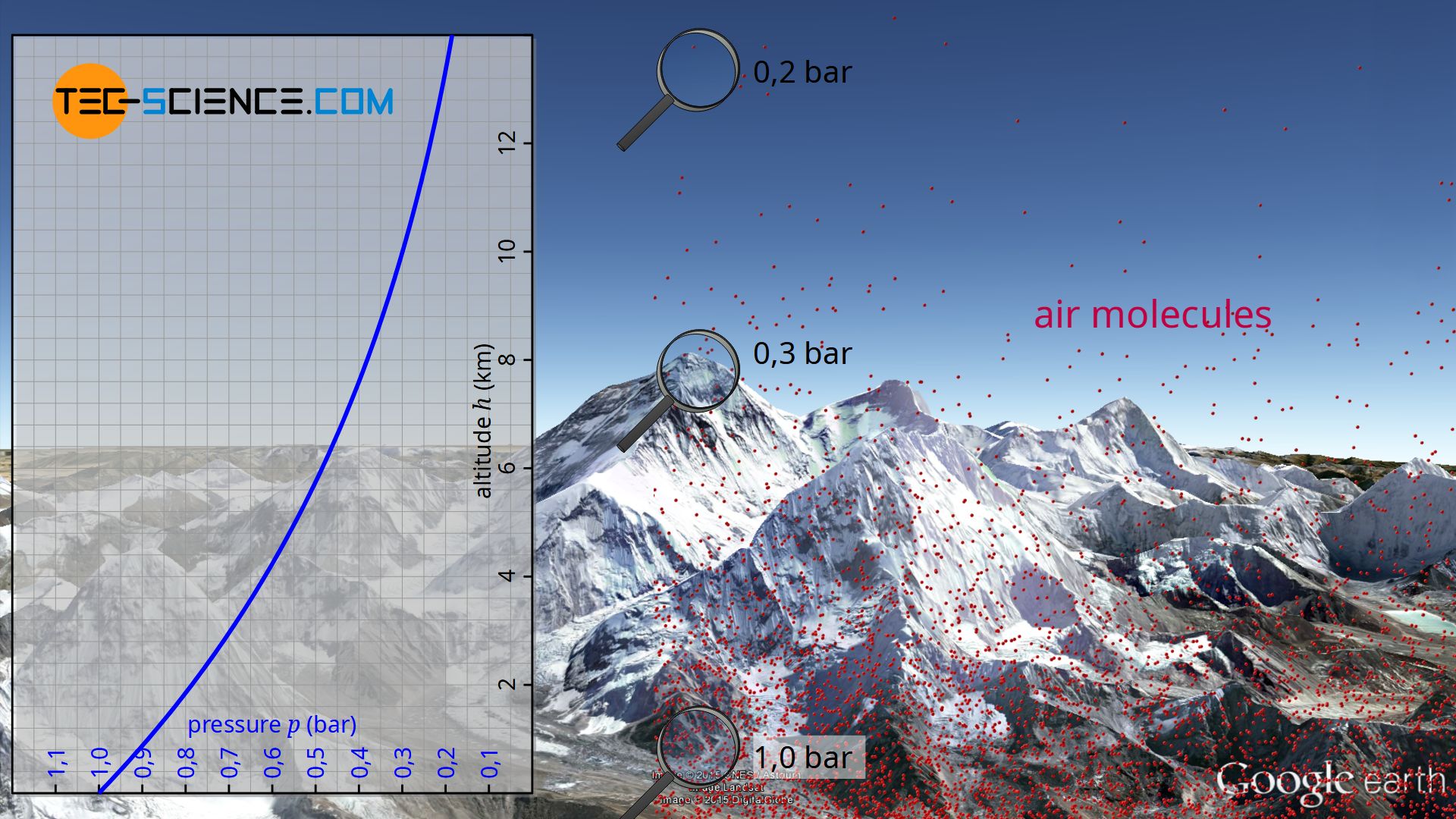
The barometric formula describes the decrease of air pressure with increasing altitude.
Introduction
At sea level the atmospheric pressure is about 1 bar. However, practice shows that the air pressure decreases more and more with increasing altitude. For example, on a mountain 2000 meters high, the atmospheric pressure is only about 0.8 bar. On Mount Everest at an altitude of 8848 meters, the air even exerts a pressure of only 0.3 bar.
With the help of the particle model, this phenomenon can be clearly understood. After all, all gas molecules have a mass, no matter how small. This leads to the fact that gas particles are also subject to gravity. There are permanent collisions between the particles and the molecules seemingly fly away in random directions. However, especially with very large dimensions, it can be seen that the air molecules are rather close to the earth’s surface due to the force of gravity. Because of this gravitational force of the earth, the air molecules are pulled down, so to speak, where they then collide with other air particles and are catapulted into the air again.
This behavior can be vividly reproduced in a model. For this purpose, balls are filled into a vertical glass tube. The glass tube stands on an oscillating plate. The vibration gives the balls a random speed. This causes the balls to collide permanently with each other, just like the molecules in the air. The balls catapult themselves to random altitudes just like the real air molecules.
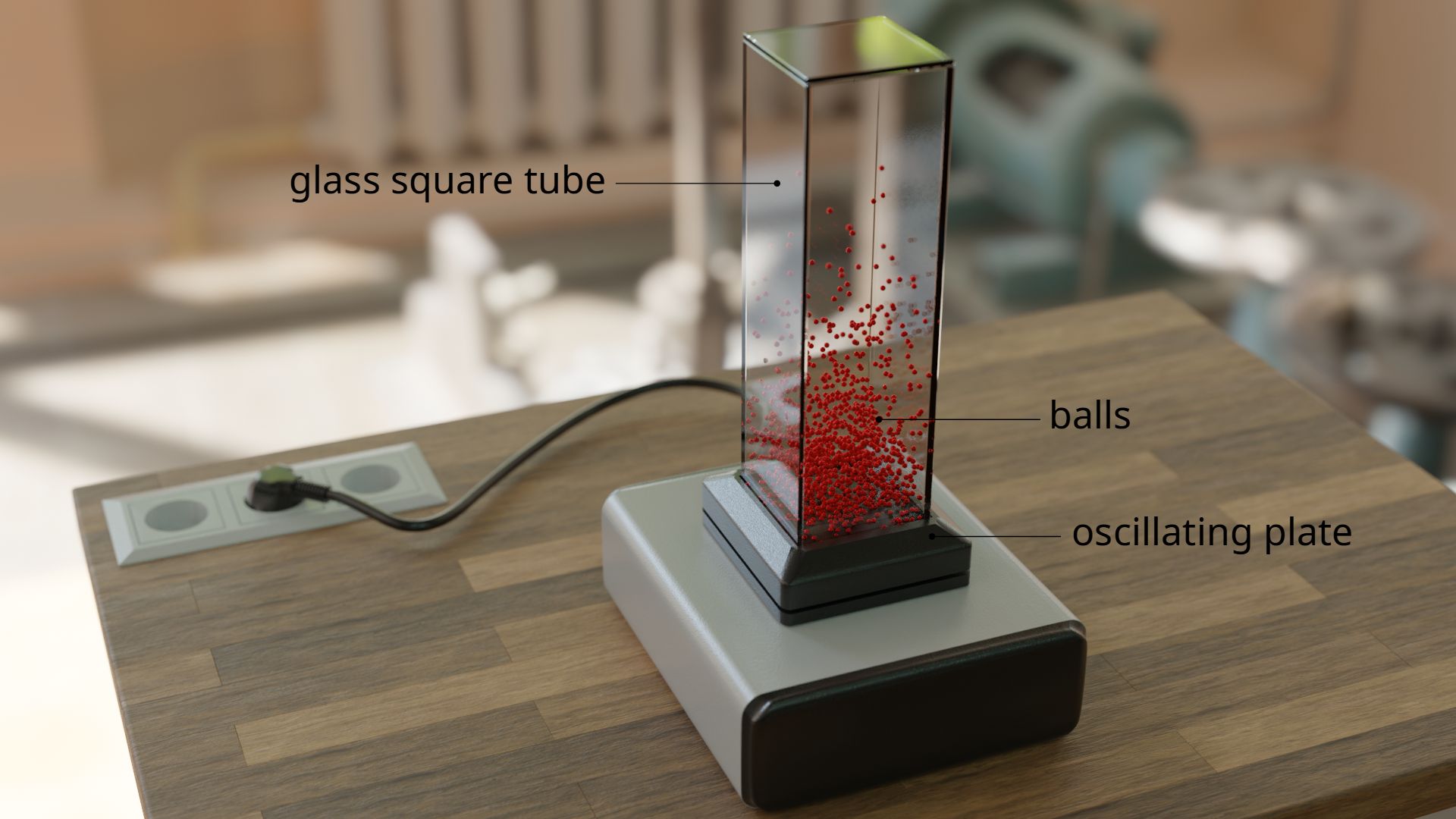
Finally, it can be observed that the majority of the balls are located near the vibrating plate. With increasing height, the density of the balls decreases, since only a few balls collide in such a way that they catapult themselves several times into the air. Such a distribution of density over the height is also found with the air molecules! The density of the air also decreases more and more with increasing altitude, as fewer and fewer air molecules can reach such great heights. This is also the reason why many climbers carry additional oxygen in compressed air bottles at high altitudes. Because the small number of air molecules is no longer sufficient to supply the body with oxygen.
The reduced air density is also directly responsible for the decreasing air pressure. In the article Pressure in gases it was already explained in detail that the pressure in gases is caused by collisions of molecules with interfaces like the a container wall or a piston. A reduced particle density thus also means a lower number of collisions. This in turn results in a lower gas pressure.
The decreasing air pressure with increasing altitude is due to the reduced air density. The decrease of the air density is due to the fact that air particles are also subject to the earth’s gravity and therefore remain more close to the earth’s surface!
Derivation of the barometric formula
The decrease in pressure with increasing altitude can be mathematically derived from a balance of forces. For this purpose, a thin layer of air with the base area A and the thickness Δh is considered at an arbitrary altitude h. The air within this layer has a certain mass Δm. One can imagine the air layer as being surrounded by an invisible envelope, i.e. as a kind of air parcel. If you consider this layer of air as being at rest, it is in equilibrium with the surrounding air. In principle, such a balance of forces would also apply if the air parcel moves up or down at a constant speed, although frictional forces would then have to be taken into account.
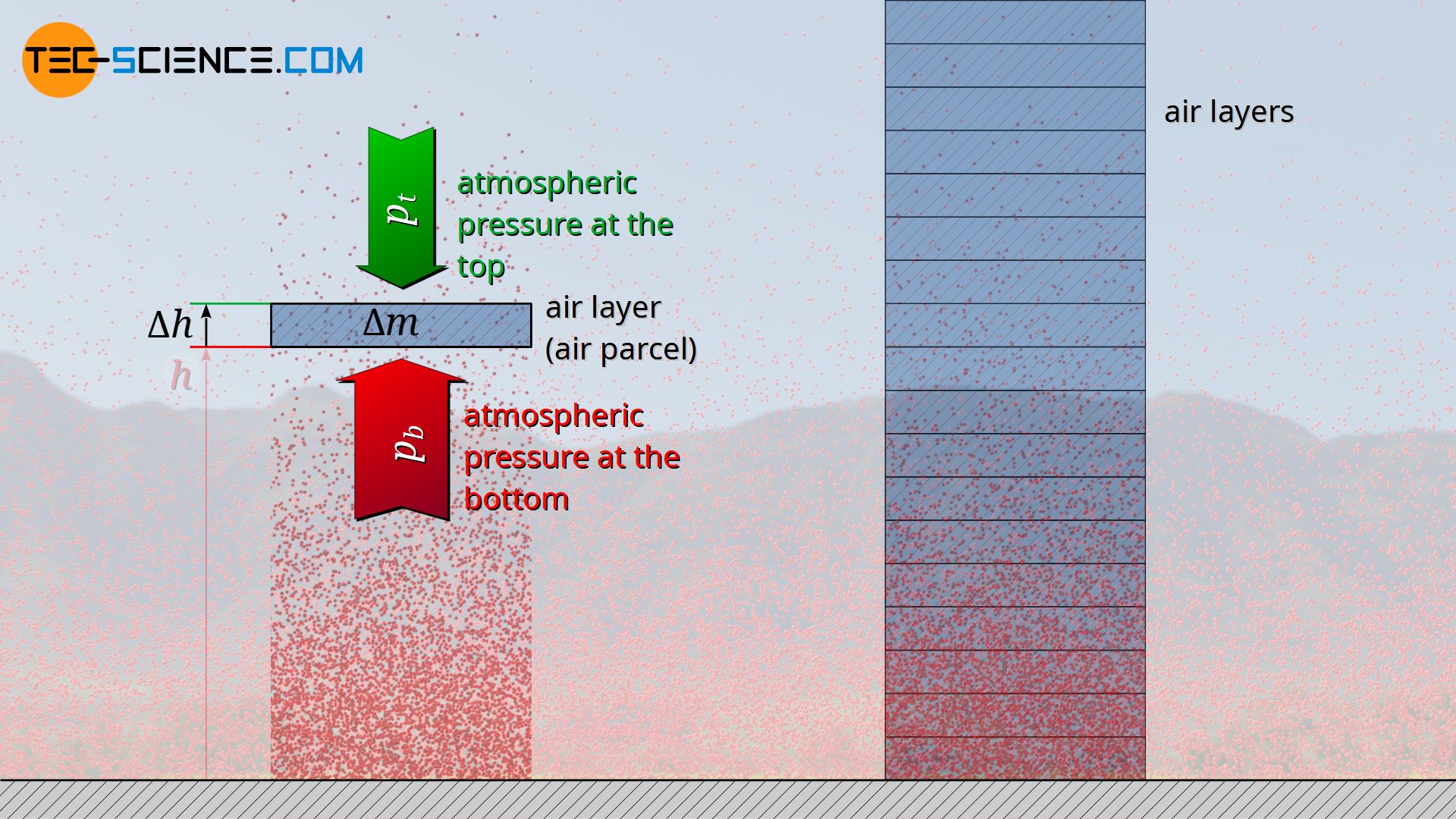
Based on this equilibrium, it is now possible to clearly understand why the air pressure below the considered air parcel must inevitably be higher than above. The surrounding air molecules exert a pressure by collisions with the air parcel both from below and from above. Only when the upward acting pressure at the bottom side is greater than the downward acting pressure at the top side, a force can effectively act upwards. This upward force counteracts the weight of the air parcel and thus keeps it stable in the air (i.e. in equilibrium).
The pressure on the bottom side of a considered air layer must be higher than on the top side, so that the weight of the air layer can be balanced by the upward force!
The atmosphere can now be imagined as being built up of countless thin layers of air. So from layer to layer, air pressure must decrease permanently, so each layer can be kept in balance by stronger forces at bottom side. For a more precise mathematical description, the forces acting on the air layers must now be examined more closely.
Relationship between change in altitude and change in pressure at a given density
For this purpose, the pressure on the bottom side of an air parcel is first examined more closely. The upward force Fb at the bottom side, which is generated by the air pressure p acting there, results from the product of the pressure and base area A of the air parcel:
\begin{align}
&F_b = p \cdot A ~~~~~\text{force at the bottom side of the air parcel} \\[5px]
\end{align}
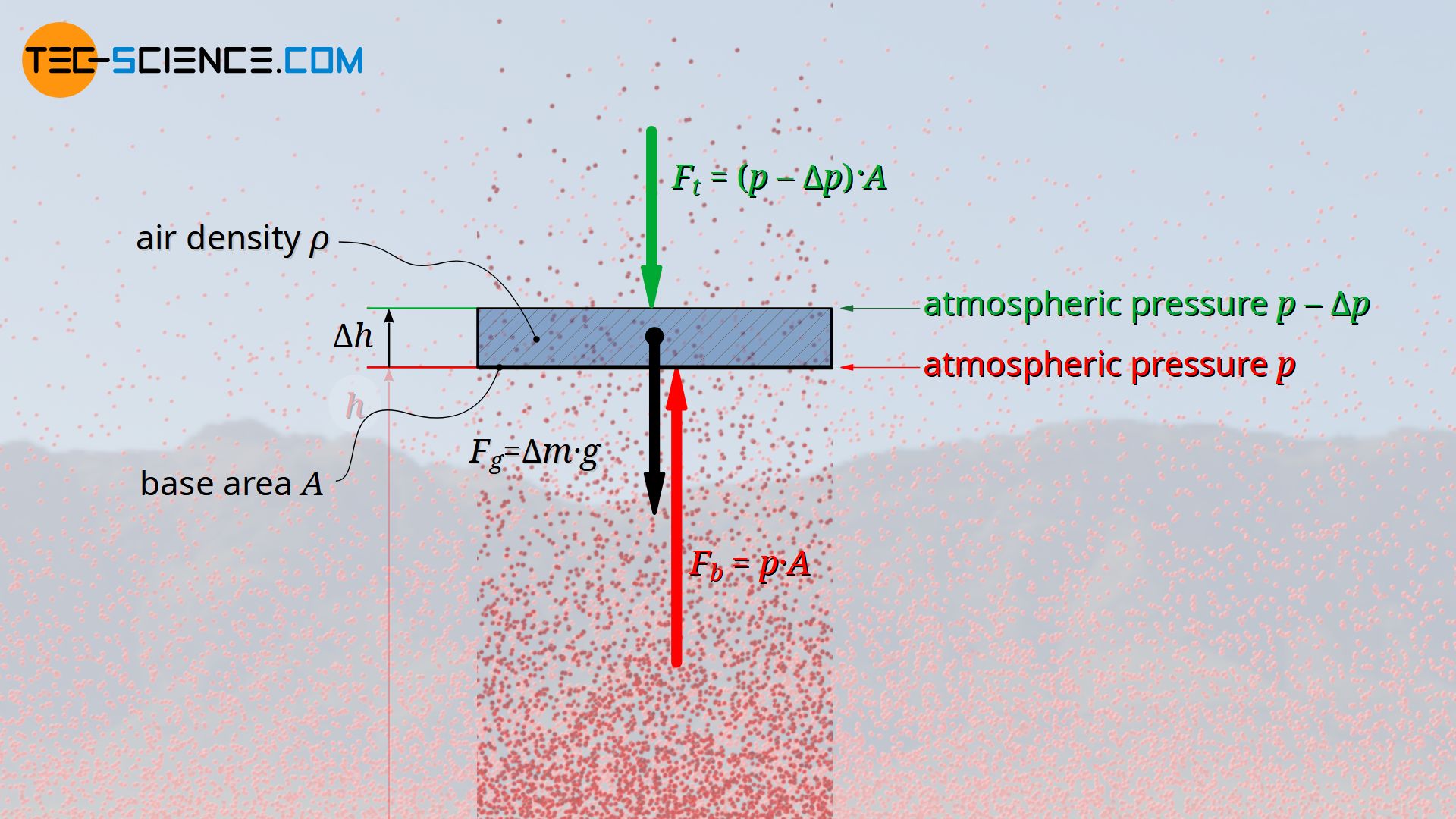
At the top of the air parcel, the air pressure will have decreased by a certain amount Δp. The downward acting force that this pressure exerts on the top of the air parcel is therefore:
\begin{align}
&F_t = \left(p-\Delta p \right) \cdot A ~~~~~\text{force at the top side of the air parcel} \\[5px]
\end{align}
The mass Δm of the air parcel can be determined from its volume ΔV and the existing air density ϱ (Δm=ΔV⋅ϱ). As already explained, the air density will decrease with increasing altitude, but since in this case only a very thin air parcel (air layer) is considered, a constant density can be assumed within this layer of air. With A as the base area and Δh as the thickness of the air layer, its weight can be calculated as follows:
\begin{align}
&F_g = \Delta m \cdot g \\[5px]
&F_g= \Delta V \cdot \rho \cdot g \\[5px]
&F_g= A \cdot \Delta h \cdot \rho \cdot g ~~~~~\text{weight of the air parcel} \\[5px]
\end{align}
The downward acting weight Fg and the also downward acting force on the top side of the air parcel Ft must now be in equilibrium with the upward acting force on the bottom side of the air parcel Fb. In this way, the following relationship can be derived between a change in altitude Δh (thickness of the air layer) and the corresponding change in pressure Δp.
\begin{align}
\require{cancel}
& F_b \overset{!}{=} F_g + F_t \\[5px]
& p \cdot \bcancel{A} = \bcancel{A} \cdot \Delta h \cdot \rho \cdot g + \left(p-\Delta p \right) \cdot \bcancel{A} \\[5px]
&\bcancel{p} = \Delta h \cdot \rho \cdot g + \bcancel{p} – \Delta p \\[5px]
\label{dp}
&\underline{\Delta p = \rho \cdot g \cdot \Delta h} ~~~~~\text{only applies to small changes in altitude }\Delta h \\[5px]
\end{align}
Note that this formula is only valid if the change in altitude Δh is not too great, since only in such a case can an (approximately) constant air density ϱ be assumed. If the change in altitude is too large, the density within the air layer is no longer constant. In this case one would have to reckon with an average air density.
Relationship between a change in altitude and a change in pressure at a given temperature
In practice, it can be assumed that within 100 meters, the air density will not change noticeably and can therefore be considered constant. If we assume that the air density near the ground is about 1.2 kg/m³, then due to the formula above there will be a change in pressure of about 12 mbar at a altitude of 100 meters:
\begin{align}
&\Delta p = \rho \cdot g \cdot \Delta h = 1.2 \tfrac{\text{kg}}{\text{m³}}\cdot 10 \tfrac{\text{N}}{\text{kg}} \cdot 100 \text{ m} = 120 \text{ Pa} = 12 \text{ mbar} \\[5px]
\end{align}
For example, if the pressure on the ground had been 1.0 bar, at an altitude of 100 meters it will have fallen by 12 mbar to a total of 0.988 bar. One could now make the same calculation for the next 100 meters, in order to calculate the pressure at an altitude of 200 meters. However, one has to keep in mind that with every difference in altitude the air density changes slightly. The air becomes thinner with increasing altitude, i.e. the air density decreases. Therefore, for each new pressure, the new air density must be taken as a basis.
This can be done, for example, by means of the ideal gas law, according to which density and pressure are directly related to each other by temperature:
\begin{align}
\label{ideal}
&\boxed{p=R_s \cdot \rho \cdot T}~~~~~\text{ideal gas law} \\[5px]
\label{rho}
&\rho=\frac{p}{ R_s \cdot T }\\[5px]
\end{align}
In this equation T denotes the thermodynamic temperature in Kelvin and Rs denotes the specific gas constant. In this case dry air with a specific gas constant of Rs=287 J(kg⋅K) is assumed. If equation (\ref{rho}) is used in equation (\ref{dp}), then the change in pressure at a given temperature can be determined:
\begin{align}
&\Delta p = \rho \cdot g \cdot \Delta h \\[5px]
&\Delta p = \frac{p}{ R_s \cdot T } \cdot g \cdot \Delta h \\[5px]
\label{dr}
&\underline{\Delta p = \frac{g}{ R_s \cdot T } \cdot p \cdot \Delta h} \\[5px]
\end{align}
Which benefit does this equation offer in comparison with the equation (\ref{dp}), because after all practice shows that not only the density but also the temperature becomes lower with increasing altitude (both unknown variables)? This is correct in principle, but the temperature changes less with increasing altitude compared to the density. For small differences in altitude the assumption of a constant temperature is therefore usually more legitimate than the assumption of a constant density!
Below an altitude of 10 km, for example, the air density decreases on average by about 1.5 % within 100 meters of altitude (temperature changes included). In comparison to this, the temperature (in Kelvin!) only drops by about 0.4 % on average. The influence of the temperature change is therefore not even one third as large as the influence of the density change. In the article Barometric formula for an adiabatic atmosphere, the influence of temperature decrease on the pressure curve is discussed in more detail.
For the sake of simplicity, however, a constant temperature will always be assumed in the following. In this context one also speaks of an isothermal atmosphere. Likewise, the decrease in gravitational acceleration with increasing altitude should not be taken into account and the specific gas constant should be regarded as constant despite any changes in atmospheric composition.
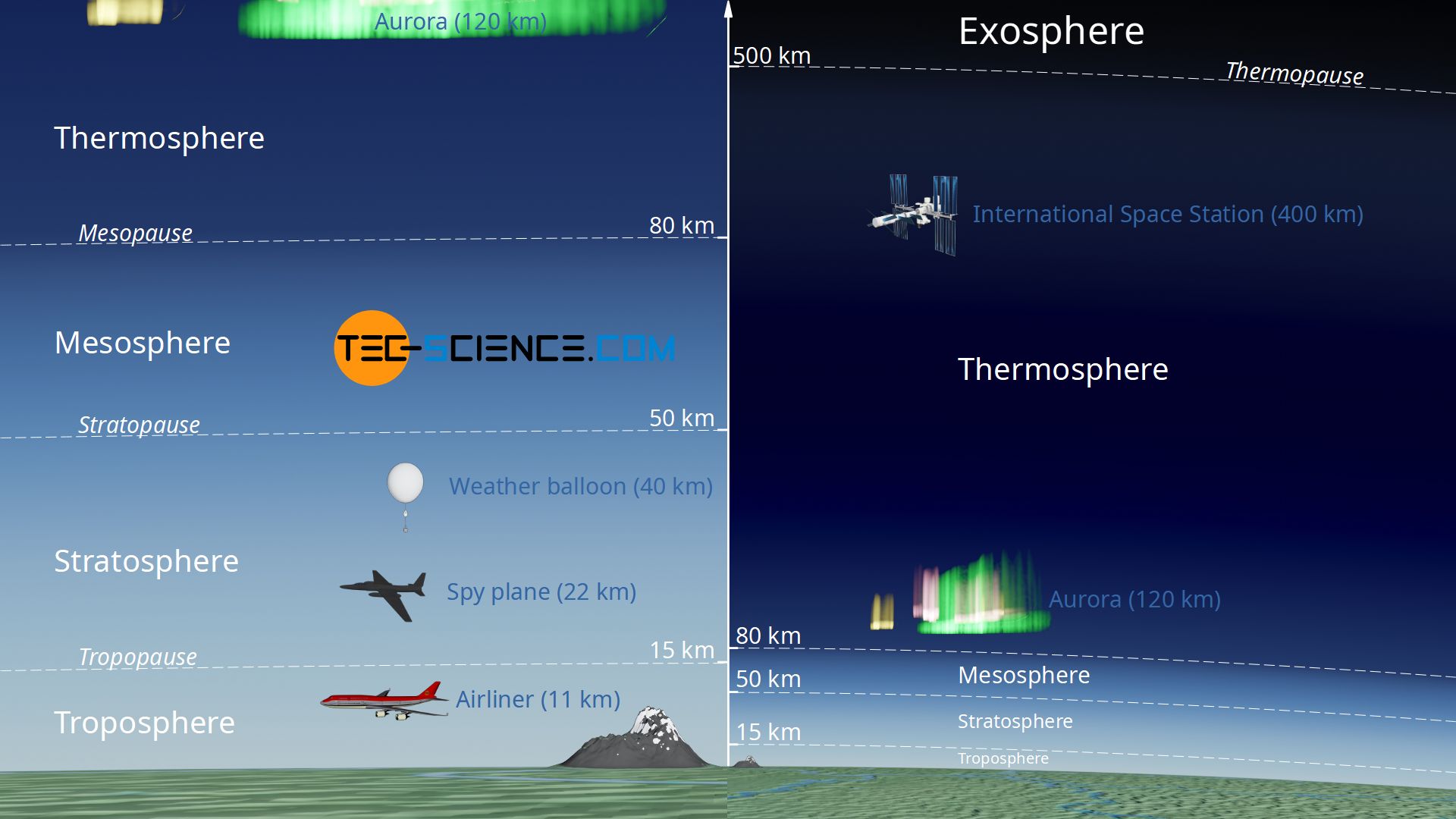
In fact, the decrease in gravitational acceleration usually plays no role in practice. Up to a height of 100 km the gravitational acceleration decreases by only 3 %. In any case, only the lower 15 km, the so-called troposphere (“weather layer”), are relevant for the weather. Also the influence of the chemical composition does not play a role below an altitude of 100 km, because the atmospheric gases are well mixed up to that point. Below an altitude of 100 km one therefore also speaks of the homosphere. The figure and animation below shows the classification of the Earth’s atmosphere into different layers. This stratification is based on the different temperatures or the characteristic temperature curves within these layers (see the article Barometric formula for an adiabatic atmosphere for more information).
When using the formula (\ref{dr}) one must always keep in mind that a positive change in altitude (Δh>0) in the mathematical sense, however, results in a negative change in pressure (Δp<0), since the pressure finally decreases with increasing altitude. For a correct mathematical definition, the upper equation must therefore still be given a negative sign. This becomes important for the barometric formula derived in the following.
\begin{align}
\label{a}
&\boxed{\Delta p = -\frac{g}{R_s \cdot T} \cdot p \cdot \Delta h} ~~~~~\text{only applies to small changes in altitude }\Delta h \\[5px]
\end{align}
Relationship between pressure and altitude change
As explained in the previous section, it is advisable to first divide the pressure change for high altitudes into small air layers of, for example, 100 meters. Then all pressure changes must be summed up to the total pressure change. However, this procedure is quite complex. The differential calculus or integral calculus offers a simpler and more general method at this point.
For this purpose, the macroscopic altitude differences are chosen smaller and smaller. In the limiting case one receives infinitesimal, i.e. infinitely small altitude differences or pressure differences. In contrast to the macroscopic changes Δh or Δp one finally obtains the corresponding differentials dh and dp. At this point at the latest, the problem with the constant density or constant temperature within the individual air layers is also resolved, since infinitely small differences in altitude are considered in the following anyway.
For the relationship between an infinitesimal height difference dh and the resulting infinitesimal pressure change dp, equation (\ref{a}) still applies:
\begin{align}
\label{c}
&\boxed{\text{d}p = -\frac{g}{R_s \cdot T} \cdot p \cdot \text{d} h} \\[5px]
\end{align}
The differential dp relating to the variable p is now rearranged to the left side of the equals sign, so that both related variables are completely separate from the differential dh on the right side of the equation (called separation of variables):
\begin{align}
&\frac{1}{p} ~ \text{d}p = -\frac{g}{R_s \cdot T} \cdot p \cdot \text{d} h \\[5px]
\end{align}
Both sides of the equation must now be integrated within the corresponding limits. Starting from a given pressure p0 at the altitude h0, the pressure p at any altitude h can be determined with the formula below. Note that temperature, gravitational acceleration and specific gas constant are assumed to be constants.
\begin{align}
&\int_{p_0}^{p}\frac{1}{p} ~ \text{d}p = \int_{h_0}^h-\frac{g}{R_s \cdot T} \cdot \text{d} h \\[5px]
&\int_{p_0}^{p}\frac{1}{p} ~ \text{d}p =-\frac{g}{R_s \cdot T} \cdot \int_{h_0}^h \text{d} h \\[5px]
&\left[\ln{\left( p\right)}\right]_{p_0}^p = -\frac{g}{R_s \cdot T} \cdot \left[~h~\right]_{h_0}^{h}\\[5px]
& \ln{(p)}-\ln{(p_0)}= -\frac{g}{R_s \cdot T} \cdot \underbrace{\left(h-h_0\right)}_{\Delta h}\\[5px]
\end{align}
The difference between the altitude h (for which the pressure p is to be determined) and the initial altitude h0 (at which the initial pressure p0 exists), corresponds to the change in altitude Δh:
\begin{align}
& \ln{(p)}-\ln{(p_0)}= -\frac{g \cdot \Delta h}{R_s \cdot T} \\[5px]
\end{align}
The left side of the equation can also be rearranged a bit. Due to algorithmic identities, the difference between two logarithmic quantities can also be expressed as the logarithmic quotient of these quantities [ln(a)-ln(b)=ln(a/b)]:
\begin{align}
& \ln{\left(\frac{p}{p_0}\right)}= -\frac{g \cdot \Delta h}{R_s \cdot T} \\[5px]
\end{align}
In order to solve this equation with respect to the pressure p, both sides of the equation are put in the exponent of the exponential function:
\begin{align}
& \text{e}^{\Large{\ln{\left(\frac{p}{p_0}\right)}}}= \text{e}^{\Large{-\frac{g \cdot \Delta h}{R_s \cdot T} }}\\[5px]
\end{align}
Since the natural logarithm (“ln”) is just the inverse function of the exponential function, the following relationship applies in general: eln(a)=a. Thus, the left hand side of the equation above corresponds to the quotient p/p0. In this way, the pressure p at a given height Δh above a reference level can be calculated with the reference pressure p0:
\begin{align}
&\frac{p}{p_0}= \text{e}^{-\frac{g \cdot \Delta h}{R_s \cdot T} }\\[5px]
\label{bar}
&\boxed{p(\Delta h)= p_0 \cdot \text{e}^{\Large{-\frac{g \cdot \Delta h}{R_s \cdot T}}}} ~~~~~\text{barometric formula} \\[5px]
\end{align}
This formula is finally called the barometric formula and indicates the pressure p as a function of altitude Δh above a reference level with known pressure p0. In comparison to the formula (\ref{a}), the barometric formula also provides a higher accuracy over larger altitudes. However, it must also be noted that this formula was derived on the assumption that neither temperature and gravitational acceleration change with altitude, nor the specific gas constant (i.e. the composition of the atmosphere). Strictly speaking, this formula is therefore also only valid for small altitudes where these conditions are met in good approximation.
With a standard pressure of p0=1.013 bar at sea level (h0=0) and a temperature of T0= 288 K (15 °C) and a specific gas constant of Rs=287 J(kg⋅K), the deviation of the barometric formula compared to the standard atmosphere below an altitude of 3 km is a maximum of 1 %. At an altitude of 6 km the deviation increases to about 5 % and continues to rise. At an altitude of 12 kilometers the deviation reaches about 27 %. The deviations are mainly due to the temperature change not taken into account.
The barometric formula can be expressed not only by temperature, but also by density at reference level. To do this, the product of the specific gas constant and temperature in the barometric formula (\ref{bar}) is replaced by the quotient of pressure and density according to the ideal gas law (\ref{bar}):
\begin{align}
&p=R_s \cdot \rho \cdot T \\[5px]
&p_0=R_s \cdot \rho_0 \cdot T \\[5px]
&\underline{R_s \cdot T = \frac{p_0}{\rho_0}} \\[5px]
\end{align}
This expression is used in equation (\ref{bar}):
\begin{align}
&p= p_0 \cdot \text{e}^{-\frac{g \cdot \Delta h}{R_s \cdot T}} ~~~~~\text{mit}~R_s \cdot T = \frac{p_0}{\rho_0} ~\text{folgt:} \\[5px]
\label{bar2}
&\boxed{p(\Delta h)= p_0 \cdot \text{e}^{\Large{-\frac{g \cdot \rho_0 \cdot \Delta h}{p_0}}}} ~~~~~\text{barometric formula} \\[5px]
\end{align}
Relationship between change in altitude and density
Since the density according to equation (\ref{rho}) is directly related to the pressure, the decrease in density with increasing altitude can also be determined using the barometric formula. For this purpose, the barometric formula (\ref{bar}) is to be used in equation (\ref{rho}):
\begin{align}
\label{x}
&\rho=\frac{p}{ R_s \cdot T}\\[5px]
\label{y}
&\rho(\Delta h)=\frac{p(\Delta h)}{ R_s \cdot T}= \frac{ p_0 \cdot \text{e}^{\Large{-\frac{g \cdot \Delta h}{R_s \cdot T}}} }{ R_s \cdot T}= \underbrace{\frac{p_0}{ R_s \cdot T}}_{\rho_0} \cdot \text{e}^{\Large{-\frac{g \cdot \Delta h}{R_s \cdot T}}} \\[5px]
\label{z}
&\boxed{\rho(\Delta h)= \rho_0 \cdot \text{e}^{\Large{-\frac{g \cdot \Delta h}{R_s \cdot T}}}} \\[5px]
\end{align}
In equation (\ref{y}) it was used that the term p0/(Rs⋅T) according to equation (\ref{x}) corresponds to the density ϱ0 at reference level. Likewise, the term Rs⋅T in equation (\ref{z}) can be replaced by p0/ϱ0:
\begin{align}
&\boxed{\rho(\Delta h)= \rho_0 \cdot \text{e}^{\Large{-\frac{g \cdot \rho_0 \cdot \Delta h}{p_0}}}} \\[5px]
\end{align}
If one compares the density course according to the barometric formula with the density course of the standard atmosphere, larger deviations of up to 7 % can be observed, especially for altitudes below 3 km. These relatively large deviations are due to the temperature, which is assumed to be constant, but which in reality decreases and thus strongly influences the density. With increasing altitude, the error made when calculating the pressure (which is the basis for calculating the density) compensates for the deviation due to the non-constant temperature. The maximum deviation of the density from the standard atmosphere is 10% at an altitude of 9 km and then decreases again.
Barometric formula vs. hydrostatic equation
One can consider the barometric formula for compressible gases in analogy to the hydrostatic equation for incompressible liquids. With increasing depth from the liquid surface, the pressure increases more and more due to the liquid layers above. In the same way, the pressure of the atmosphere increases when moving from high altitudes towards the earth’s surface. We live at the bottom of a “sea of air”, so to speak. The only difference between the gas of the atmosphere and the liquid of the seas is that liquids cannot be compressed. This means that the pressure in liquids increases (almost) without an increase in density, whereas in gases the density also increases with increasing pressure.
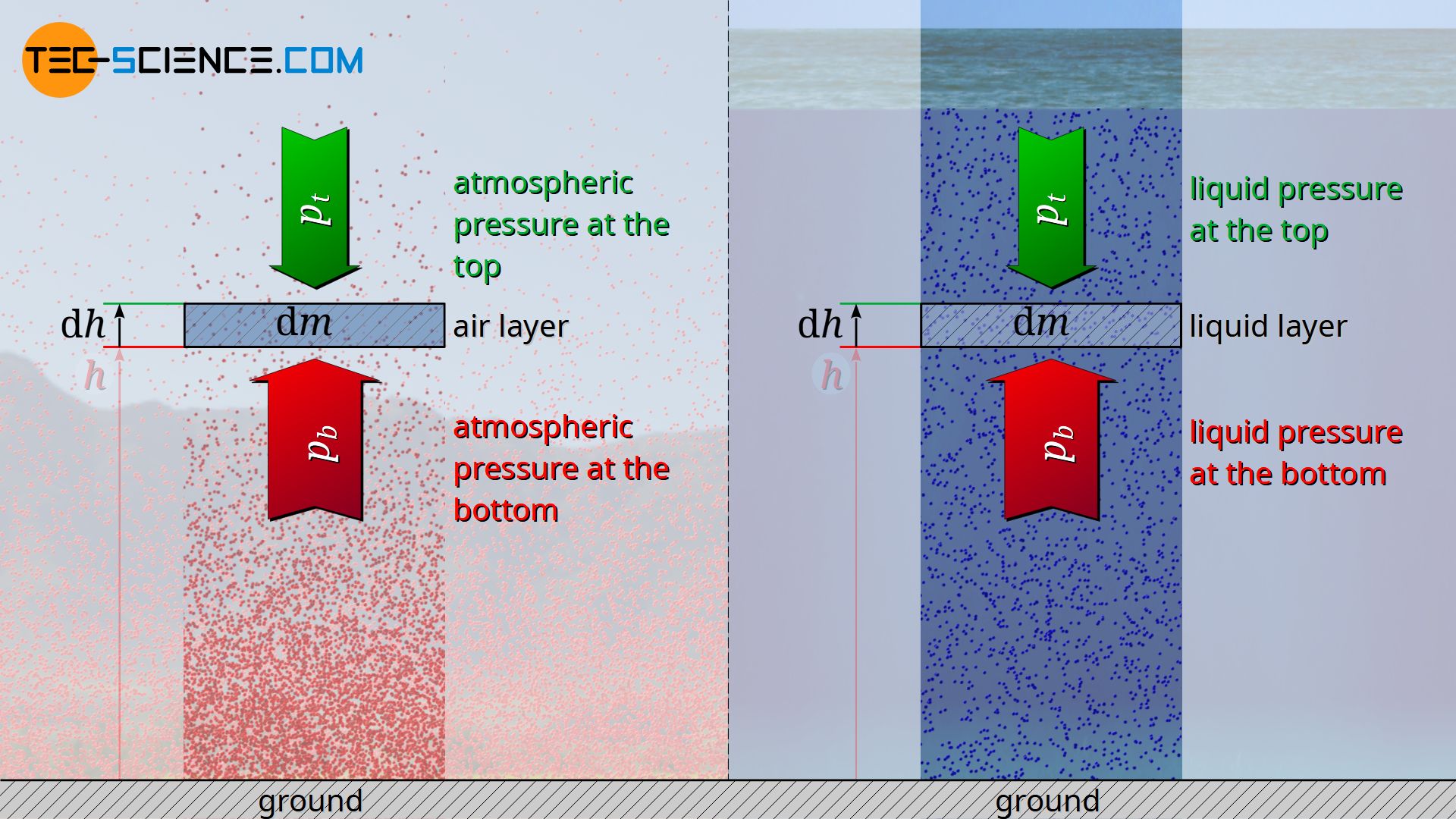
The hydrostatic equation for incompressible fluids can also be derived from the previous considerations. For this purpose equation (\ref{dp}) is to be used for infinitesimal changes. Then this equation can be integrated with the assumption of a constant density ϱ.
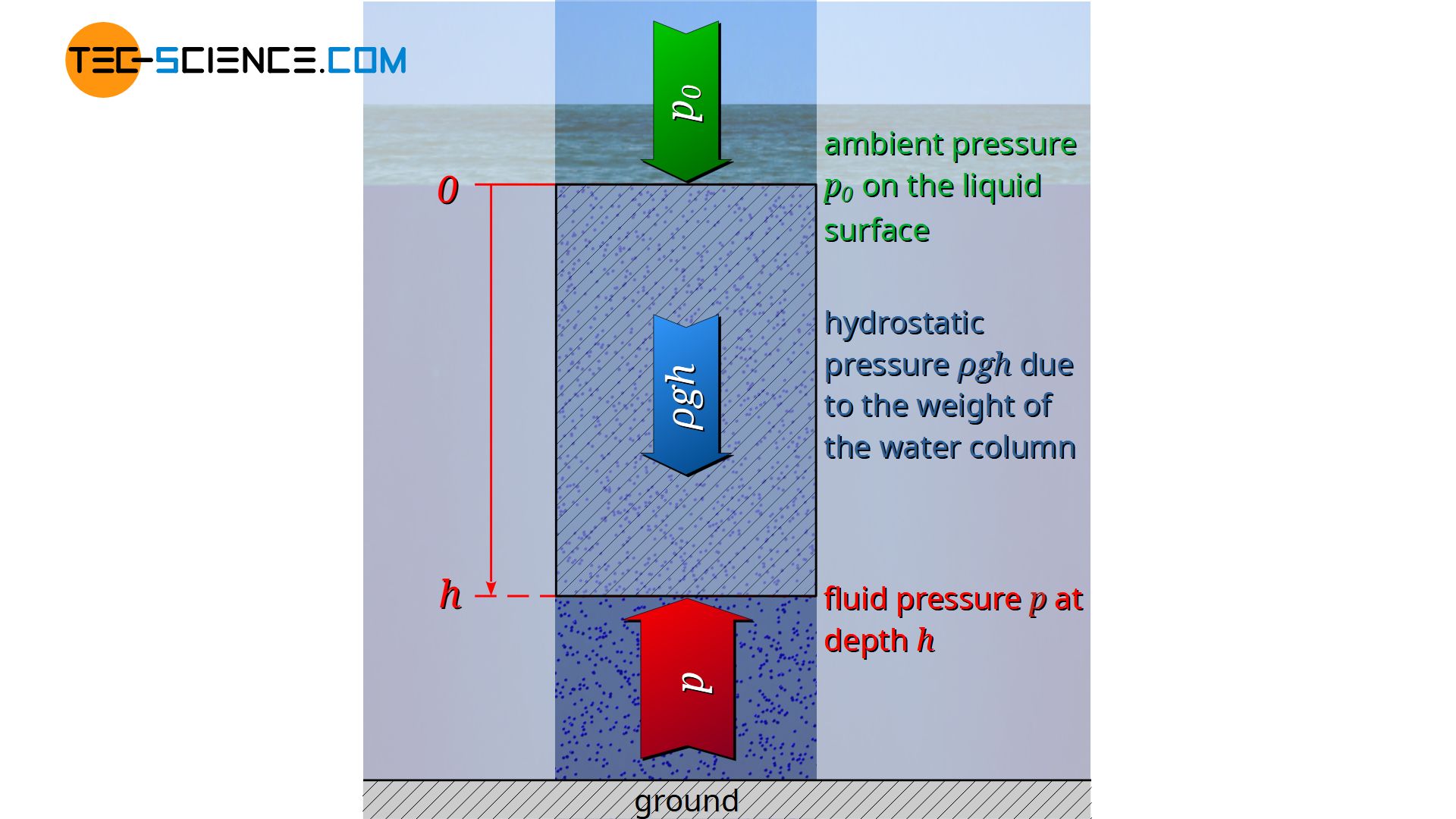
For practical reasons, the liquid surface rather than the bottom of the liquid is taken as the reference level. The integration limits are therefore based on the liquid surface h=0 on which the ambient pressure p0 is applied, down to the depth h with the liquid pressure p to be determined there:
\begin{align}
\text{d}p &= \rho \cdot g \cdot \text{d} h \\[5px]
\int_{p_0}^{p} ~ \text{d}p & = \rho \cdot g \cdot \int_{0}^{h} \text{d} h \\[5px]
\left[p\right]_{p_0}^{p} &= \rho \cdot g \cdot \left[h\right]_{0}^{h} \\[5px]
\left[p-p_0\right] &= \rho \cdot g \cdot \left[h-0\right] \\[5px]
\underbrace{p}_{\text{total pressure}} &= \underbrace{p_0}_{\text{ambient pressure}} + \underbrace{\rho \cdot g \cdot h}_{\text{hydrostatic pressure}}
\end{align}
\begin{align}
\boxed{p=p_0 + \rho g h} ~~~~~\text{hydrostatic equation}\\[5px]
\end{align}
The derived hydrostatic equation states that the pressure in liquids results from the sum of ambient pressure and hydrostatic pressure.