Solidification can be triggered either by nuclei consisting of the same substance as the melt or by nuclei consisting of a different substance.
Introduction
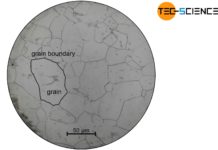
As explained in the article on solidification conditions, the presence of nuclei is a basic requirement for liquids to solidify. The forming of a nucleus is called nucleation. In principle, the nucleation can take place in two different ways. Either as heterogeneous nucleation or homogeneous nucleation.
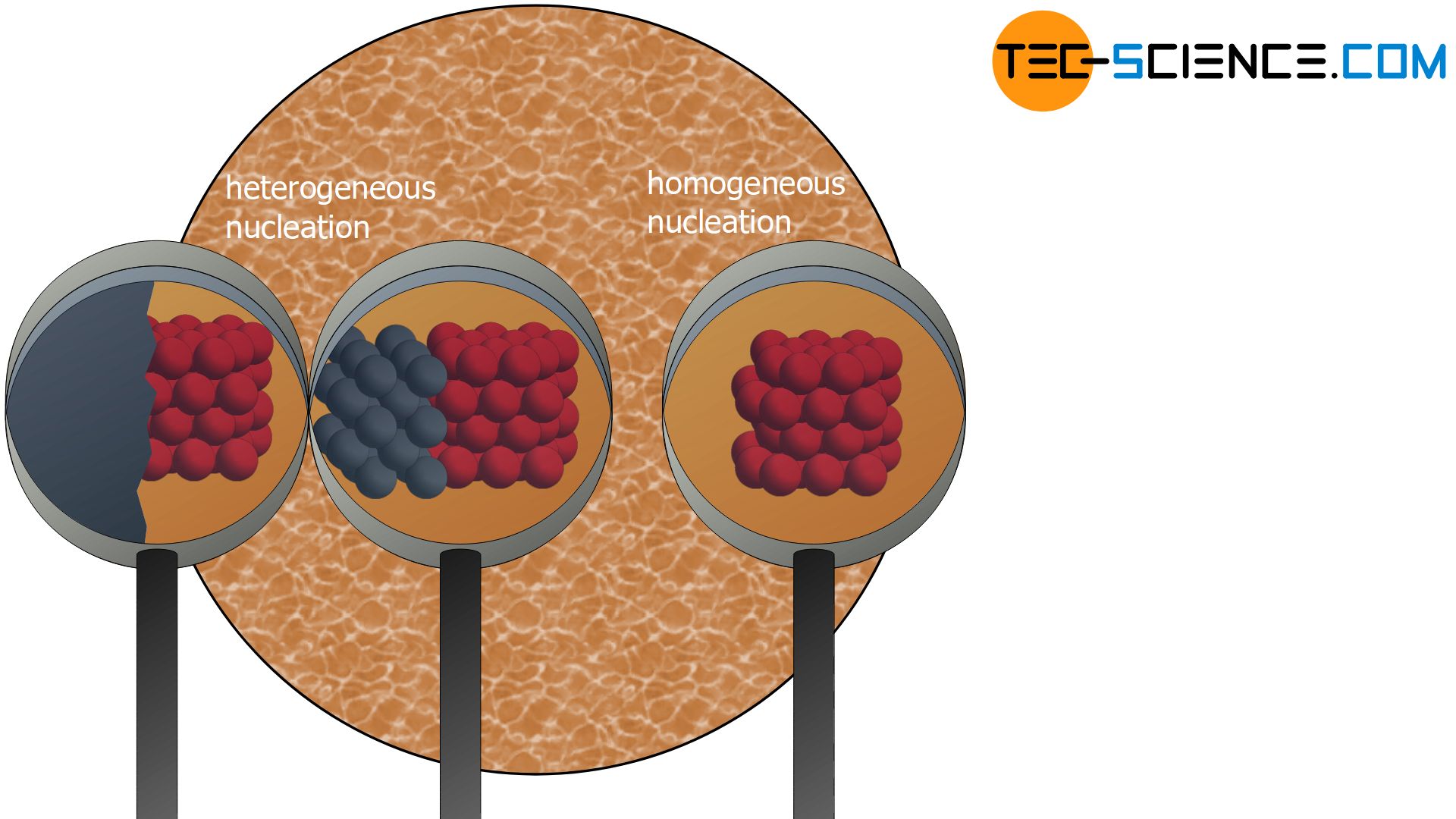
Heterogeneous nucleation
If foreign particles serve as nuclei (i.e. particles of a different substance than the ones of the melt itself), one also speaks of heterogeneous nuclei. Heterogeneous nuclei can be caused by impurities in the melt. But the vessel walls in which the melt is held also serve as heterogeneous nuclei with their foreign particles.
Such foreign particles are the most common reason in everyday life why undercooled liquids are generally not observed and one could therefore prematurely conclude that only the condition of falling below the solidification temperature (melting point) would be necessary for a solidification process.
The formation of heterogeneous nuclei preferentially occur at places where the foreign particles are located (e.g. on the vessel wall). The probability of nucleation is therefore not homogeneously distributed over the melt but concentrates on the contaminated areas. This is the reason why it is called “heterogeneous” nucleation.
Nucleation caused by foreign substances is referred to as heterogeneous nucleation!
Homogeneous nucleation
Not only foreign particles but also the own particles of the substance can serve as nuclei. In this context one also speaks of homogeneous nucleation.
Homogeneous nuclei can be formed, for example, by the atoms arranging themselves randomly in the melt in the form of an unit cell or lattice structure. Due to the large number of particles in a melt, the formation of such homogeneous nuclei is not as unlikely as it may sound at first. Homogeneous nuclei can also be caused by unmelted residues, which is, however, rather unlikely in molten metals.
The probability of a random arrangement of atoms in the form of a lattice structure is homogeneously distributed over the entire melt. So there are no preferred places where the particles come together. This is the reason why it is called “homogeneous” nucleation.
The nucleation caused by own particles is called homogeneous nucleation!
Influencing the nucleation
With the knowledge of the necessary prerequisites for the solidification process, the formation of microstructures can now be specifically controlled.
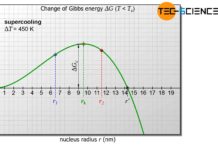
Thus, with increasing undercooling (supercooling), the probability that the melt will form heterogeneous nuclei increases to a certain extent. After all, strong supercooling means greater inertia of the particles and thus the probability that self-formed nuclei will dissolve decreases.
A large undercooling usually leads to an increased homogeneous nucleation. The melt then begins to solidify at many nuclei simultaneously. This creates a very fine-grained microstructure with many grain boundaries, which is characterised by very good strength and toughness.
The stronger the supercooling, the more nuclei form in the melt and the finer the resulting grains!
Another possibility to achieve a fine-grained structure is the targeted admixture of foreign particles, which then serve as heterogeneous nuclei. This process is called seeding.
For the seeding of nodular cast iron, for example, a compound of iron and silicon is often used. However, since the added foreign particles tend to dissolve in the melt after some time, the seeding process should take place immediately before solidification or during the casting of the metal.
A fine-grained structure can also be achieved by seeding the melt with specifical foreign particles!
The formation of the microstructure can basically be divided into two phases: nucleation and nucleus growth. Both the phase of nucleation and the phase of nucleus growth can be specifically influenced in order to shape the structure according to the properties desired. Due to their complexity, these phases are dealt with in more detail in separate articles.