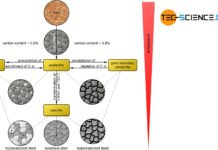
With increasing carbon, the hardness and strength of unalloyed steels increases. Above a content of 0.8% C, the strength decreases.
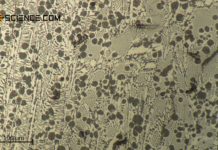
As the carbon content increases, the proportion of cementite in the steel also increases. Since the cementite is relatively hard, the hardness of the steel increases accordingly. This results in an almost linear relationship between the carbon content and the hardness of the (unalloyed) steel.
Furthermore, the fine-lamellar cementite precipitation in the microstructure makes the dislocation movement more difficult, which results in a corresponding increase in strength (see hardening mechanisms). The fine lamellas serve as “barriers” for the migrating dislocations, so to speak. Since more cementite is precipitated with increasing carbon content, the fraction of fine lamellar pearlite structure also increases. As the carbon content increases, so does the strength of the steel.
From a carbon concentration of 0.8 %, however, additional precipitation of cementite takes place at the grain boundaries, which in turn leads to embrittlement. This of course only applies to unalloyed steels, i.e. steels that contain no other alloying elements apart from carbon. However, additional alloying elements such as chromium, nickel, manganese, titanium, etc. can also significantly increase strength beyond a carbon content of 0.8 %.
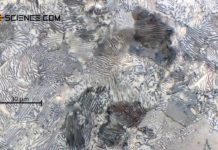
The strength of the steel is therefore decisively determined by the lamellar cementite structure. The finer the pearlite microstructure, the higher the strength. A very fine lamellar structure can be achieved by increased undercooling. It should be noted, however, that as the cooling rate increases, no thermodynamic equilibrium can ultimately be established between or within the phases. Both the transformation temperatures and the types of microstructures that occur change and the iron-carbon phase diagram loses its validity in its actual form.
Phase diagrams only apply at (infinitely) slow cooling speeds!
This means, for example, that a rapid cooling during the \(\gamma\)-\(\alpha\)-transformation leaves no time for the carbon to diffuse out. The result is a new distorted microstructure called martensite. This is used, among other things, for hardening and tempering steel.