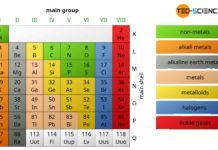
Substances can be categorized into different groups, such as pure substances or mixtures, depending on their structural composition.
First, you can distinguish between pure substances and mixtures. Pure substances contain only one type of particle. In the simplest case, particle means a chemical element. For example hydrogen (H), pure iron (Fe) or graphite (carbon C). Not only single atoms but also whole molecules can form pure substances. These substances are characterized by a certain atomic ratio and are referred to as chemical compounds. Pure compounds for example are water (H2O), carbon dioxide (CO2), acetone (C3H6O) or cementite (Fe3C).
In contrast to mixtures, pure substances have fixed atomic ratios (only in the specific case of elements does a pure substance consist of one type of atom)!
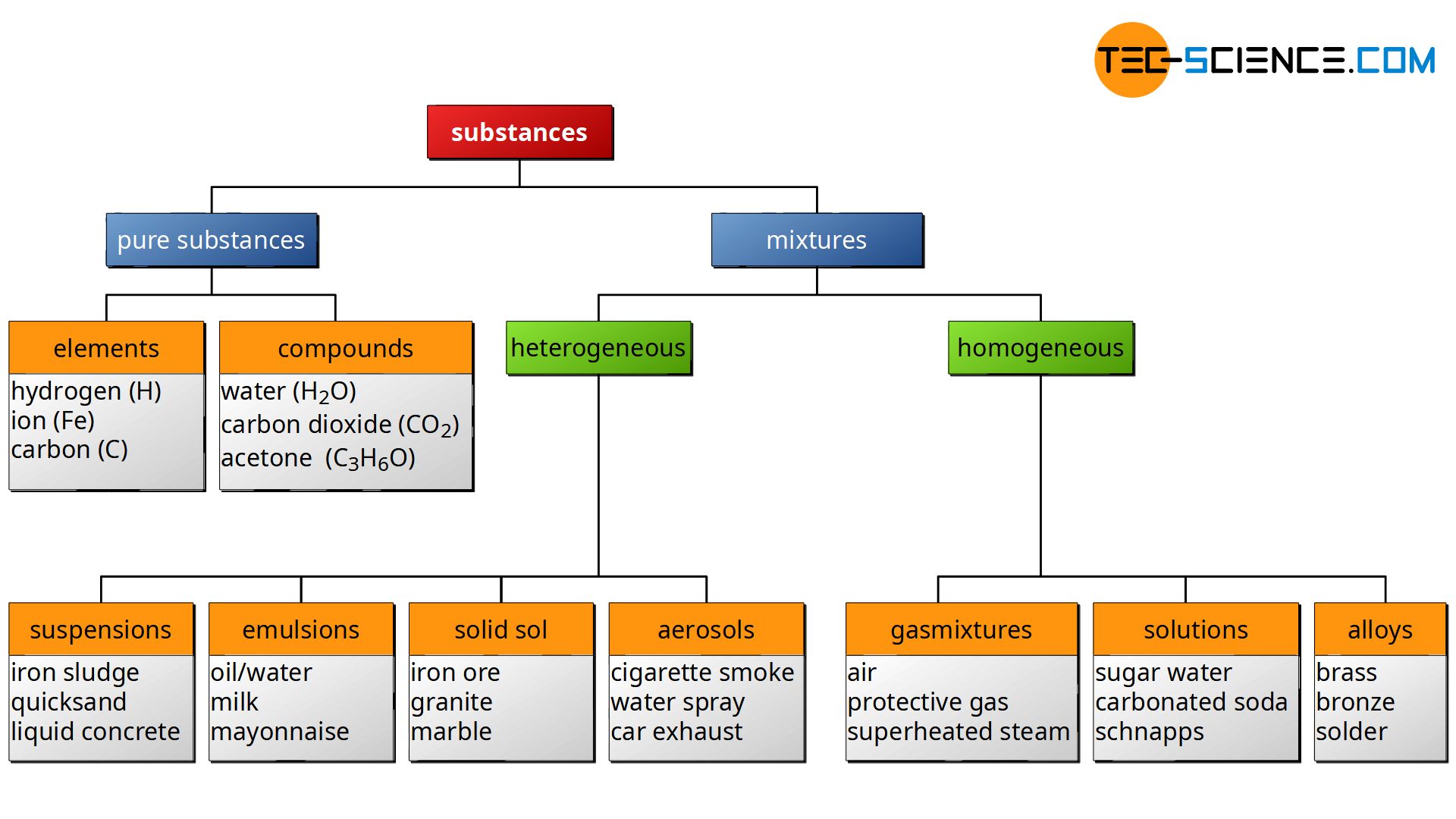
If matter consist of several types of particles (atoms or molecules) with no specific atomic ratio due to chemical bonds, this will be referred to as mixtures. Such mixtures can be further classified into heterogeneous mixtures and homogeneous mixtures.
Homogeneous mixtures do have a uniform distribution of the different particles. Homogeneous mixtures for example are gasmixtures like air as well as solutions. But in contrast to a gas mixture, the state of matter of a solution is liquid. Therefore homogeneous mixtures can further by classified into gasmixtures and solutions. The group of solutions includes, for example, sugar water, salt water or carbonated soda. In addition to gases and liquids, solids can also form homogeneous mixtures. This is the case with some alloys such as copper-nickel alloys. So this could be a third classification of homogeneous mixtures.
Mixtures of substances with an diverse distribution of the containing particles are referred to as heterogeneous mixtures. A mixture of a solid and a liquid is called a suspension, for example, iron sludge, quicksand or liquid concrete. Heterogeneous mixtures of different liquids, which can not be mixed homogeneously, will be referred to as emulsions (e.g. oil-water-mixture, milk, mayonnaise, etc.). For heterogeneous mixtures of two or more solids, such as iron ore, granite or marble, one speaks of a solid sol. The last group of heterogeneous mixtures are the aerosols. These are mixtures of solids or liquids in gases. Examples of aerosols are cigarette smoke, water fog or car exhaust.
A suspension is a mixture of solid particles dissolved in a liquid. A mixture of two different liquids, which can not be dissolved homogeneously, is called emulsion.