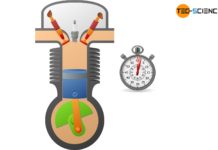
Polytropic process in a closed system
Learn more about polytropic thermodynamic processes in closed systems in this article.
Particular processes shown in a volume-pressure diagram
The figure below shows the course of...
Derivation of the formulas for work and heat of a polytropic process
In this article you will learn more about the derivation of the formulas for calculating work, heat and change of internal energy for polytropic...
Derivation of the formulas of the isentropic “adiabatic” process
In this article, learn more about the derivation of the formulas and equations describing the isentropic (adiabatic) process.
Basic equations
For the derivation of the equations...
Isentropic (“adiabatic”) process in a closed system
An isentropic process is a reversible process of an adiabatic system.
Definition
Whereas in an isochoric process no pressure-volume work is done by the system or...
Isothermal process in a closed system
In this article, learn more about the calculation of pressure, volume, work and heat in an isothermal process in a closed system.
A change of...
Isobaric process in a closed system
In this article, learn more about the calculation of volume, temperature, work, and heat in an isobaric process in a closed system.
A change of...
Isochoric process in a closed system
In this article, learn more about the calculation of pressure, temperature, work and heat in an isochoric process in a closed system.
A change of...
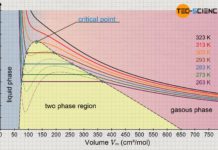
Explanation of liquefaction using the Van der Waals equation
In this article, learn how the Van der Waals equation can be used to explain the liquefaction of gases at high pressures.
Van der Waals...
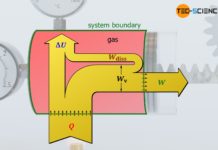
What is meant by dissipation of energy?
Dissipation is the (partial) conversion of a certain form of energy into thermal energy that cannot be fully converted back into the original form...
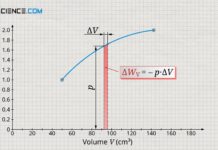
Concept of pressure-volume work (displacement work)
The pressure-volume work (displacement work) is the work don on the gas or by the gas due to the acting gas pressure during a...
Dissipation of energy in closed systems
Learn in this article why, in thermodynamic processes with dissipation of energy, the pressure-volume work of the gas does not correspond to the work...
Derivation of the pressure-volume work (displacement work)
Learn in this article why the area under the curve in a volume-pressure diagram corresponds to the pressure-volume work (displacement work).
In the article concept...
Why do pressure and temperature increase during the compression of a gas?
The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in...
Van der Waals equation (gas law for real gases)
The Van der Waals equation describes the relationship between pressure, volume and temperature for real gases.
Ideal gas law
In thermodynamic processes, gases are often considered...
Internal energy of ideal gases
In ideal gases, the change in internal energy is directly related to the change in temperature. Learn more about the relationships in this article.
Simplified...
Calculation of the internal energy for ideal gases
Learn more about calculating the internal energy for ideal gases in this article.
First law of thermodynamics
In the article Internal energy of ideal gases it...
Law of Gay-Lussac for ideal gases (Charles’s law)
The law of Gay-Lussac describes the relationship between an increase in temperature and the resulting increase in volume at constant pressure (isobaric process).
Isobaric process
If...
Law of Amontons for ideal gases
The law of Amontons describes the relationship between an increase in temperature and the resulting increase in pressure at constant volume (isochoric process).
Isochoric process
If...
Law of Boyle-Mariotte for ideal gases
The law of Boyle-Mariotte describes the relationship between a decrease in volume and the resulting increase in pressure at constant temperature (isothermal process).
Isothermal process
If...
The process quantities: Heat and work
Work and heat are process quantities that describe the process of a supply of energy ("energy in transit")! Learn more about it in this...
Internal energy & first law of thermodynamics
Internal energy is the sum of the different forms of energy on a microscopic level inside a substance. Learn more about it in this...
Thermodynamic systems
A thermodynamic system is a confined space of matter (e.g. gas) within which thermodynamic processes take place. The system boundary separates the system from...
Ideal gas law (explained and derived)
The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal gases.
Parameters influencing the gas pressure
The figure below shows a...