Derivation of the Maxwell-Boltzmann distribution function
The Maxwell-Boltzmann distribution function of the molecular speed of ideal gases can be derived from the barometric formula.
Introduction
For ideal gases, the distribution function f(v)...
Equipartition theorem
The equipartition theorem states that the kinetic energy of the gas molecules is equally divided along all three spatial directions!
Equipartition theorem
In the article Pressure...
Determination of the speed distribution in a gas
Learn more about experimentally determining the velocity distribution of molecules in gases in this article.
Introduction
As already explained in the article Temperature and particle motion,...
Pressure and temperature (kinetic theory of gases)
In this article, learn more about the relationship between pressure and temperature in connection with the kinetic theory of gases.
Introduction
In order to connect the...
Maxwell–Boltzmann distribution
The Maxwell-Boltzmann distribution describes the distribution of the molecular speed of the molecules in ideal gases.
Introduction
As already explained in the article Temperature and particle...
Why do liquids evaporate?
In this article, learn how the evaporation of liquids can be qualitatively explained using the Maxwell-Boltzmann distribution.
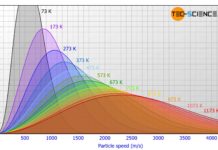
Maxwell-Boltzmann distribution of ideal gases
The figure below shows...