Why steam burns are more dangerous than water burns?
Steam burns are more dangerous than water burns because more heat is transferred due to the additional release of latent heat of condensation.
To vaporize...
Why does water extinguish fire?
By absorbing a very large amount of heat during vaporization, water draws energy from the fire site and thus cools it down until the...
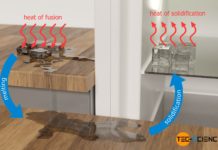
Specific latent heat of solidification (enthalpy of solidification)
Specific heat of solidification is the heat energy to be released for solidification of a liquid per kilogram of the substance!
Melting and solidification
In the...
Specific latent heat of fusion (enthalpy of fusion)
The specific latent heat of fusion (enthalpy of fusion) is the amount of heat required to melt a solid substance!
Process of melting
If a solid...
Specific latent heat of condensation
Specific heat of condensation is the heat energy to be released for condensation of a gas per kilogram of the substance!
Vaporization and condensation
In the...
Specific latent heat of vaporization
The specific latent heat of vaporization (enthalpy of vaporization) is the amount of heat required to vaporize a liquid substance!
Process of vaporization
If a liquid...
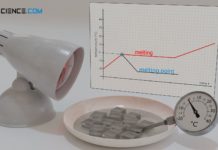
Why does the temperature remain constant during a change of state (phase transition)?
During a change of the state of matter, the supplied energy is not used to increase the kinetic energy of the molecules, but to...
Final temperature of mixtures (Richmann’s law)
Richmann's law of mixtures describes the final temperature resulting in thermodynamic equilibrium when two bodies with different initial temperatures are brought into contact.
Adiabatic mixing
If...
Heating and cooling of several objects
Learn more about calculating the final temperature of several objects with different temperatures in this article.
Heating of several objects
In practice, when heating or cooling...
Heat capacity of objects
Heat capacity is the amount of heat required to raise the temperature of an object by 1 Kelvin (1 °C). Learn more about it...
Calorimeter to determine the specific heat capacities of liquids
Calorimetry deals with the measurement of heat energy.These measurements are based on temperature changes, which are used to determine the amount of heat involved.
Test...
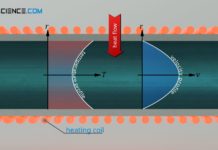
Specific heat capacity of gases (at constant volume or pressure)
Due to compressibility of gases, a distinction must be made between the isobaric and the isochoric specific heat capacity.
Differentiation between isochoric and isobaric heat...
Specific heat capacity of water
The specific heat capacity of water depends on the temperature and is strongly dependent on the state of matter.
The specific heat capacity is not...
Specific heat capacity of selected substances
In this article, learn more about the specific heat capacity of different materials and how it affects the change in temperature over time during...
Important remarks on the specific heat capacity
https://www.youtube.com/watch?v=0JZOK2hcQok
Definition of the specific heat capacity
The specific heat capacity c describes the relationship between a transfer of heat Q and the associated temperature change...
Specific heat capacity (derivation and definition)
The specific heat capacity indicates how much heat must be absorbed by a substance of mass 1 kg in order to increase its temperature...
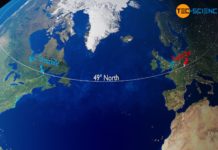
Gulf Stream & global ocean conveyor belt
The Gulf Stream is an ocean current in the Atlantic Ocean which, as part of the earth's global conveyor belt, has a decisive influence...
Difference between thermal conductivity, diffusivity, transmittance, resistance and heat transfer coefficient
Learn more in this article about the differences and importance of thermal conductivity, thermal diffusivity, heat transfer coefficient, thermal transmittance and thermal resistance, etc.
Thermal...
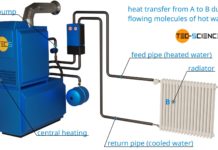
Why are radiators usually located under a window?
Learn in this article, why radiators are usually located under a window?
Central heating systems use the principle of thermal convection. The water is heated...
Dimensionless numbers of the boundary layers (Prandtl, Schmidt and Lewis number)
To describe the heat and mass transport, dimensionless numbers are introduced to describe the processes within the boundary layers.
Between a flowing fluid and a...
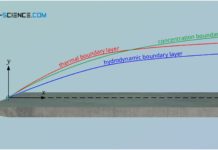
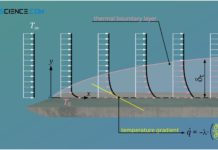
Thermal and concentration boundary layer
In addition to the hydrodynamic boundary layer, the thermal boundary layer and the concentration boundary layer also have a decisive influence on the entire...
Prandtl number
The Prandtl number is a dimensionless similarity parameter to describe the transport of heat and momentum.
Definition
In the article on the different boundary layers, the...
Lewis number
The Lewis number is a dimensionless similarity parameter to describe heat and mass transport.
The Lewis number always comes into play when a flowing fluid...
Calculation of the Nusselt numbers for forced flows over plates and in pipes
In this article you will find formulas for calculating the local and average Nusselt numbers for forced flows over plates and in pipes with...
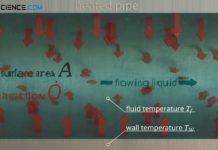
Heat transfer coefficient for thermal convection
The heat transfer coefficient describes the convective heat transfer from a solid to a flowing fluid and vice versa!
Introduction
The heat transfer coefficient describes the...
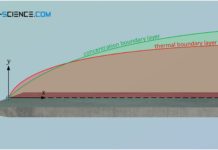
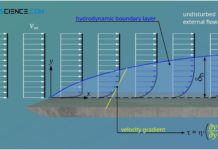
Hydrodynamic boundary layer
The hydrodynamic boundary layer of a flow has a decisive influence on heat and mass transport.
Introduction
In this article we take a closer look at...
Nusselt number to describe convective heat transfer
The Nusselt number is a dimensionless similarity parameter to describe convective heat transfer, independent of the size of the system.
Introduction
Convective heat transfer describes the...
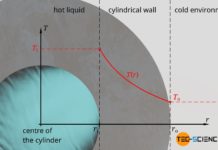
Temperature profiles and heat flows through different geometries
In this article we discuss temperature curves and heat flows through a plane wall, through a cylindrical pipe and through a hollow sphere.
Introduction
Temperature differences...
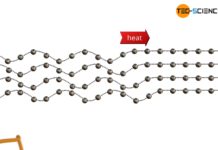
Thermal conduction in solids and ideal gases
The thermal conductivity in crystalline, non-metallic solids first increases and then decreases again with increasing temperature.
Phonons: Quasiparticles of the lattice vibrations
Thermal conduction refers to...
Laser-Flash method for determining thermal conductivity (LFA)
With the Laser-Flash method (Laser Flash Analyser, LFA), the thermal conductivity is determined by the temperature rise in a test sample that is heated...
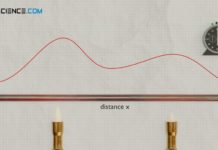
Transient-Hot-Wire method method for determining thermal conductivity (THW)
With the Transient-Hot-Wire method (THW), the thermal conductivity is determined by the change in temperature over time at a certain distance from a heating...
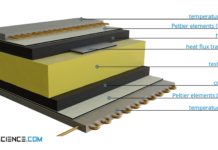
Heat-Flow-Meter method for determining thermal conductivity (HFM)
With the Heat-Flow-Meter method (HFM) the thermal conductivity is determined by comparative measurement of the heat flow using a reference sample.
Thermal conductivity
Thermal conductivity is...
Guarded-Hot-Plate method for determining thermal conductivity (GHP)
With the Guarded-Hot-Plate method (GHP) the thermal conductivity is determined by the electrical power output of a hot plate with guided heat conduction.
Thermal conductivity
Thermal...
Derivation of heat equation (diffusion equation)
The heat equation describes the temporal and spatial behavior of temperature for heat transport by thermal conduction.
Derivation of the heat equation
We first consider the...
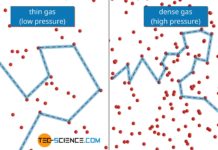
Thermal conductivity of gases
The thermal conductivity of ideal gases is not dependent on pressure for gases that are not too strongly diluted. This is no longer the...
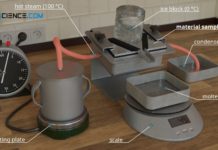
Experimental setup for determining thermal conductivity
In this article you can learn more about the experimental determination of the thermal conductivity of materials using steam and ice.
Thermal conductivity
Thermal conductivity is...
Thermal transmittance (U-value)
The thermal transmittance (U-value or U-factor) describes the heat transfer through a solid object, which is located between two fluids (gas or liquid) with...
How does a thermos work? Design of a vacuum flask!
Learn more about the structure of a vacuum flask and how a thermos works in this article!
The reason why hot tea or coffee stays...
Heat transfer by thermal radiation
With thermal radiation, heat is transferred by electromagnetic waves without the presence of a substance!
The mechanisms of thermal convection and thermal conduction explained in...
Heat transfer by thermal convection
With heat transfer by thermal convection, heat is transported with a flowing substance. Convection only occurs in fluids, i.e. gases and liquids.
Introduction
One possibility of...
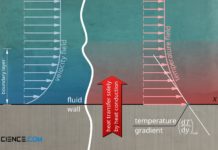
Heat transfer by thermal conduction
Heat transfer by thermal conduction means that heat is conducted through a material. Heat energy is transferred from molecule to molecule at the atomic...
Rate of heat flow: Definition and direction
The rate of heat flow refers to the heat energy transferred per unit of time (heat output). The drive for the heat flow is...
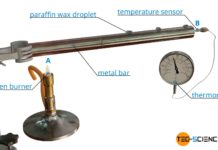
Thermal conductivity (Fourier’s law)
Thermal conductivity is a measure of how well or poorly a material conducts heat energy (measure of the strength of heat conduction)!
Thermal conduction
In general,...
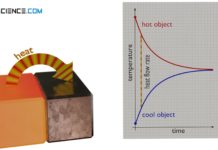
Heat and thermodynamic equilibrium
In thermodynamics, heat is the transport of energy due to a temperature difference. Heat in this respect is never "contained" in an object!
Equalization of...
Heat transfer (heat transport)
Heat transfer is the transport of thermal energy from a warmer object to a cooler object. A distinction is made between convection, conduction and...
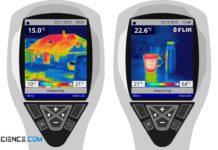
Why does metal feel colder than wood (human thermal response)?
Find out in this article why metal feels colder than wood of the same temperature, while at higher temperatures the metal suddenly feels warmer...