Density anomaly refers to the paradoxical behavior of a substance to expand suddenly when cooling down instead of contracting further (anomalous decrease in density).
Negative thermal expansion (density anomaly)
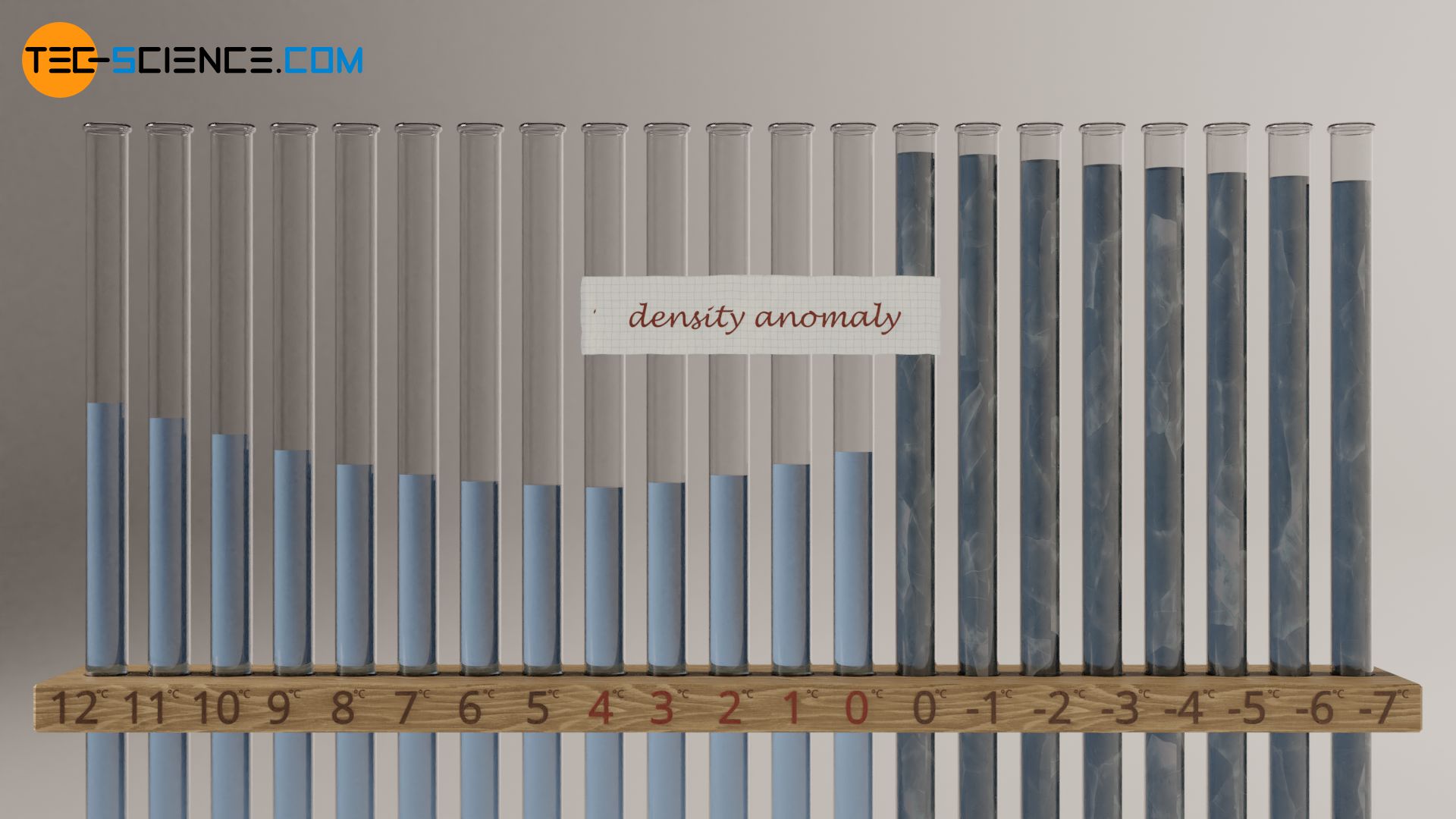
Due to its special molecular structure, water behaves differently than most other substances during cooling. In order to illustrate this, several test tubes are shown in the figure below, each filled identically with water. Now the water is cooled down more and more. At first, water contracts more and more due to the decreasing Brownian motion. As expected, the volume of the water decreases and the density increases. Note that volume and density are reciprocal to each other.

Below 4 °C, however, water behaves differently than most liquids. The volume does not decrease as usual as it cools down further, but increases. The density decreases accordingly in this temperature range. Let’s look at the situation the other way round, i.e. in the case of heating: When water is heated in the range between 0°C and 4°C, the water contracts instead of expanding. That’s why this phenomenon is known as negative thermal expansion (NTE) or density anomaly. At 4 °C (more precisely: 3.98 °C) water has the smallest volume or its highest density of 0.99997 g/cm³! Such a negative thermal expansion does not only occur with water, but also with other substances such as silicon or germanium.
Negative thermal expansion (density anomaly) refers to the paradoxical behavior of a substance to contract when heated instead of expanding!
The density anomaly is crucial for life on earth! Among other things, the anomaly causes ice to form on the surface of a lake. Thus, the water under the ice layer usually remains liquid, allowing the fish to survive in winter.
Cause of negative thermal expansion
The reason for negative thermal expansion of water are so-called clusters. These are accumulations of water molecules that are held together by hydrogen bonds (H-bonds). However, such clusters can only form stable at relatively low temperatures. At too high temperatures, the Brownian motion is so intense that the clusters are torn apart again due to the weak hydrogen bonds. Conversely, the intensity of particle motion of the water molecules decreases with decreasing temperature, so that more water molecules can accumulate on clusters without being torn away immediately.
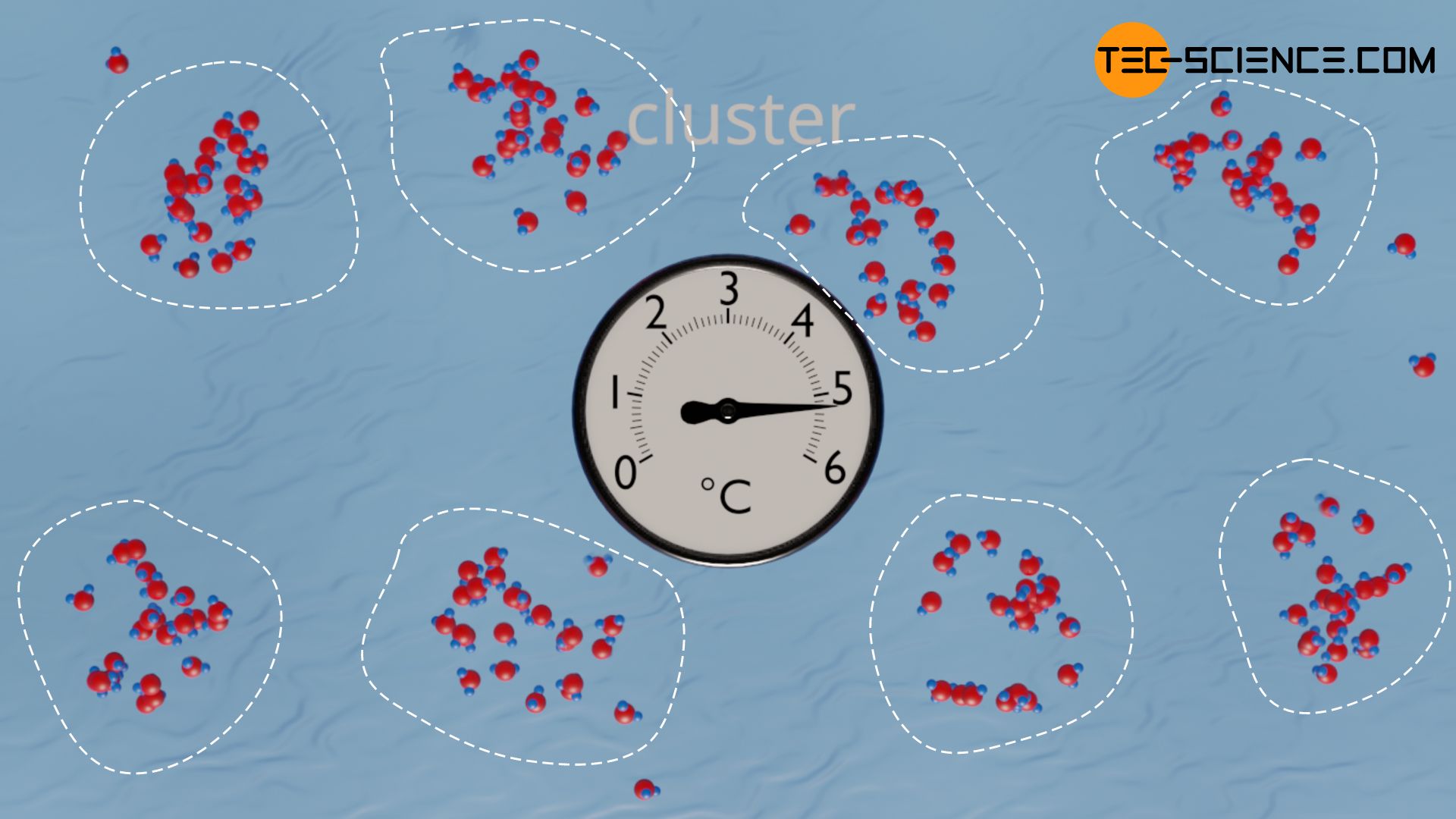
The clusters thus become larger and larger as the temperature drops, and the space required by the clusters increases accordingly. At the same time, however, the individual clusters move closer together due to the effect of thermal contraction. The latter effect dominates at first, so that water contracts as expected during cooling.
As the temperature drops further, the volume of the clusters increases disproportionately and gradually compensates for the effect of thermal contraction. At 3.98 °C, the effect of the growing clusters is as great as the effect of thermal contraction. At this point, the water has the smallest volume or the highest density. If the temperature drops further below 3.98 °C, the effect of the growing clusters exceeds the effect of thermal contraction and the water volume increases again.
With further cooling near the solidification point, the clusters become larger and larger and the hydrogen bonds become more stable or less unstable. The clusters therefore form crystal-like structures of solid ice. However, these clusters are not yet stable and dissolve after a short time (the hydrogen bonds are only stable when the water movement of the molecules is literally frozen). In principle, the lifetime of the individual clusters in this unstable state is usually only a few trillionths of a second. While such clusters quickly disintegrate in one place, water molecules accumulate again in another place to form new clusters.
Increase in density during solidification
If the water is cooled further, it finally solidifies at 0 °C to form ice with stable hydrogen bonds. During this phase transition hexagonal structures are formed due to the angled H2O molecules. In the middle of these structures is a lot of “empty” space. This leads to the fact that the molecules in the solid state (ice) take up a much larger volume than in the liquid state. Therefore the density decreases strongly during solidification. This abrupt increase in volume when solidifying is also a peculiarity of water, which is caused by the density anomaly!
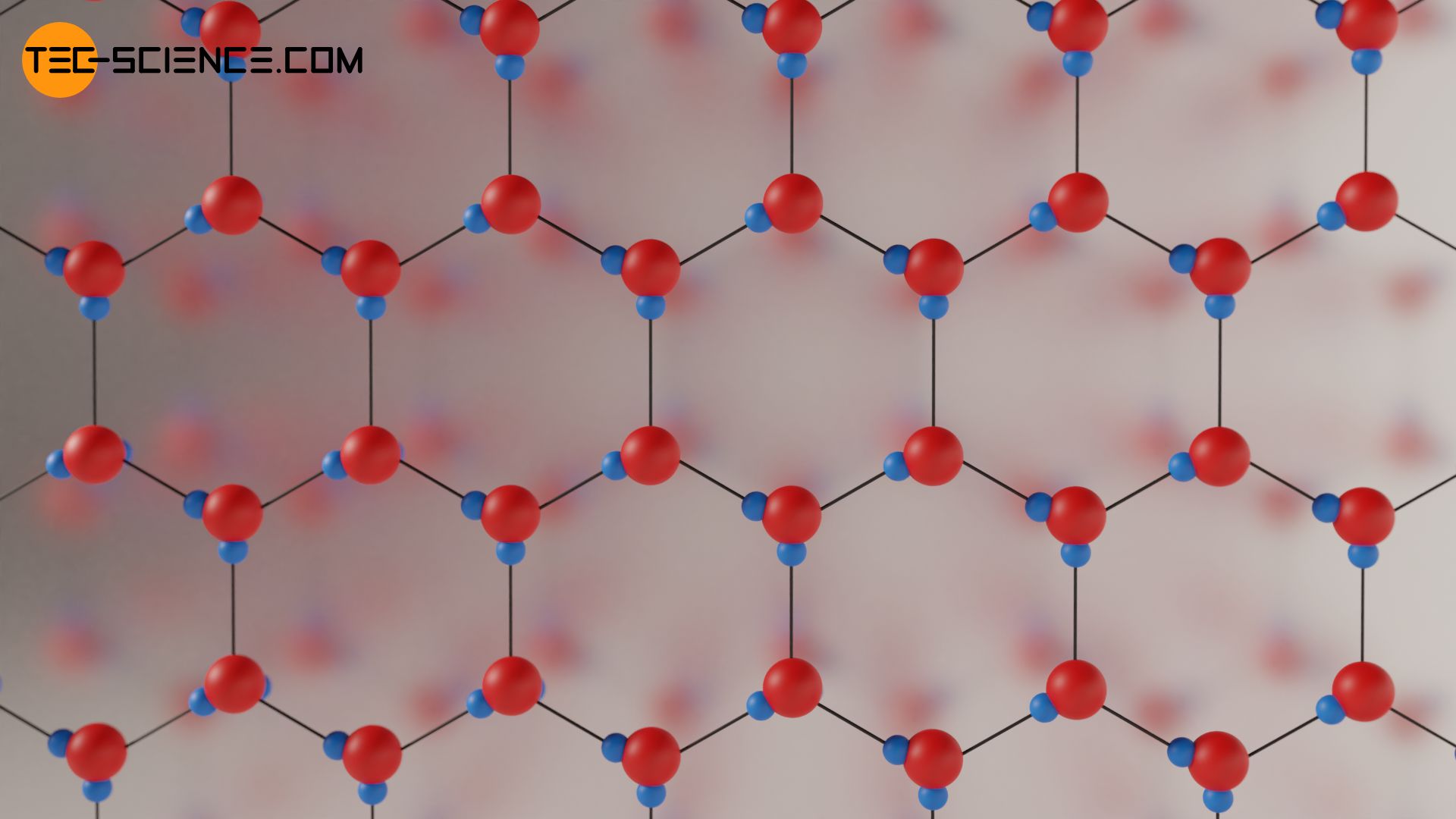
While liquid water at 0°C has a density of 1.000 g/cm³, the density at the same temperature in the solid state is only 0.917 g/cm³. The volume of the water therefore increases by about 9 % during freezing. Drink bottles should therefore never be filled to the brim when they are placed in the freezer. Otherwise the bottle would burst due to the strong expansion of the water during freezing!
The forces acting due to the increase in volume can even cause entire boulders to break when water penetrates the cracks and freezes in the cavities. The increase in volume is also the reason why, over time, frozen water causes potholes in the asphalt.
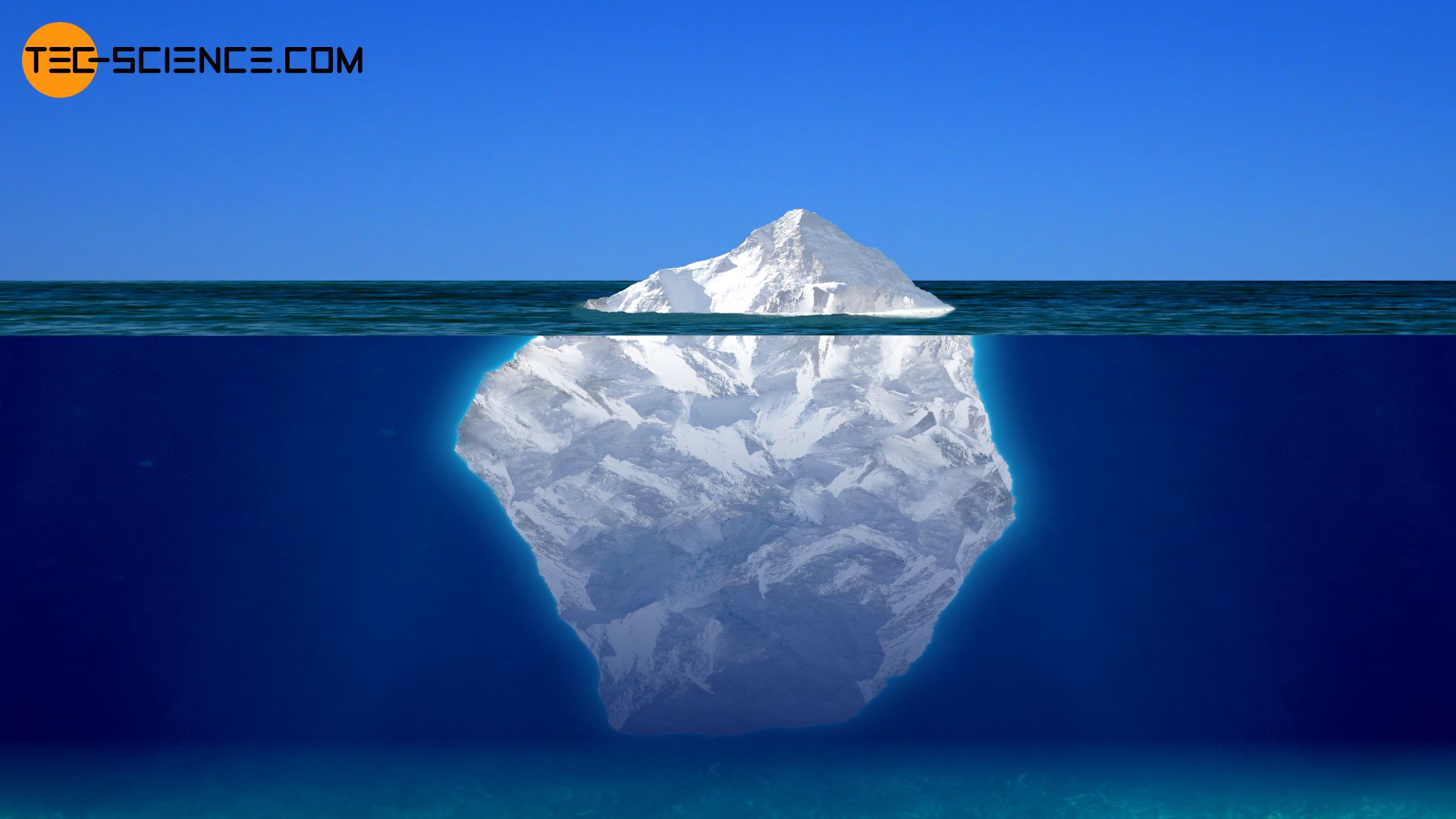
Due to the density anomaly, ice is lighter than water of the same volume. This is the reason why ice cubes in a glass of water are floating or icebergs in the sea float on the sea surface.

Since the density of ice is 8.3 % lower than that of liquid water, theoretically only 8.3 % of the ice is above the surface of the water. However, this does not take into account the fact that seawater, for example, has a higher density than fresh water because of the salt dissolved in it. This provides additional buoyancy to the iceberg, which usually consists of fresh water. It must also be remembered that an iceberg does not normally form a homogeneous mass but contains air pockets or dirt. As a result, icebergs will sink less into the water than theoretically calculated. Nevertheless, it can be assumed that under these considerations no more than about 10 to 20% of the iceberg will be above the water surface.