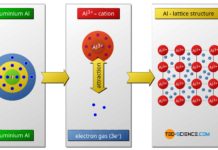
In ionic bonding, the metal atoms give off their outer electrons, which are taken up by the non-metal atoms.
The ionic bond is the predominant type of bonding between a metal and a nonmetal. The metal atoms involved in the binding release their valence electrons, which are taken up by the nonmetal atoms. In both cases, the noble gas configuration for the respective atoms is achieved.
The metal atom becomes a positively charged ion (cation) after the release of the electrons. The non-metal atom becomes a negatively charged ion (anion) after the electrons are taken up. The cohesion between the metal and non-metal atoms is due to the electrostatic attraction of the resulting ions. The ionic bond has special significance for ceramics.
In an ionic bond, the metal atoms release their valence electrons, which are taken up the nonmetal atoms in order to reach the noble gas configuration for each atom!
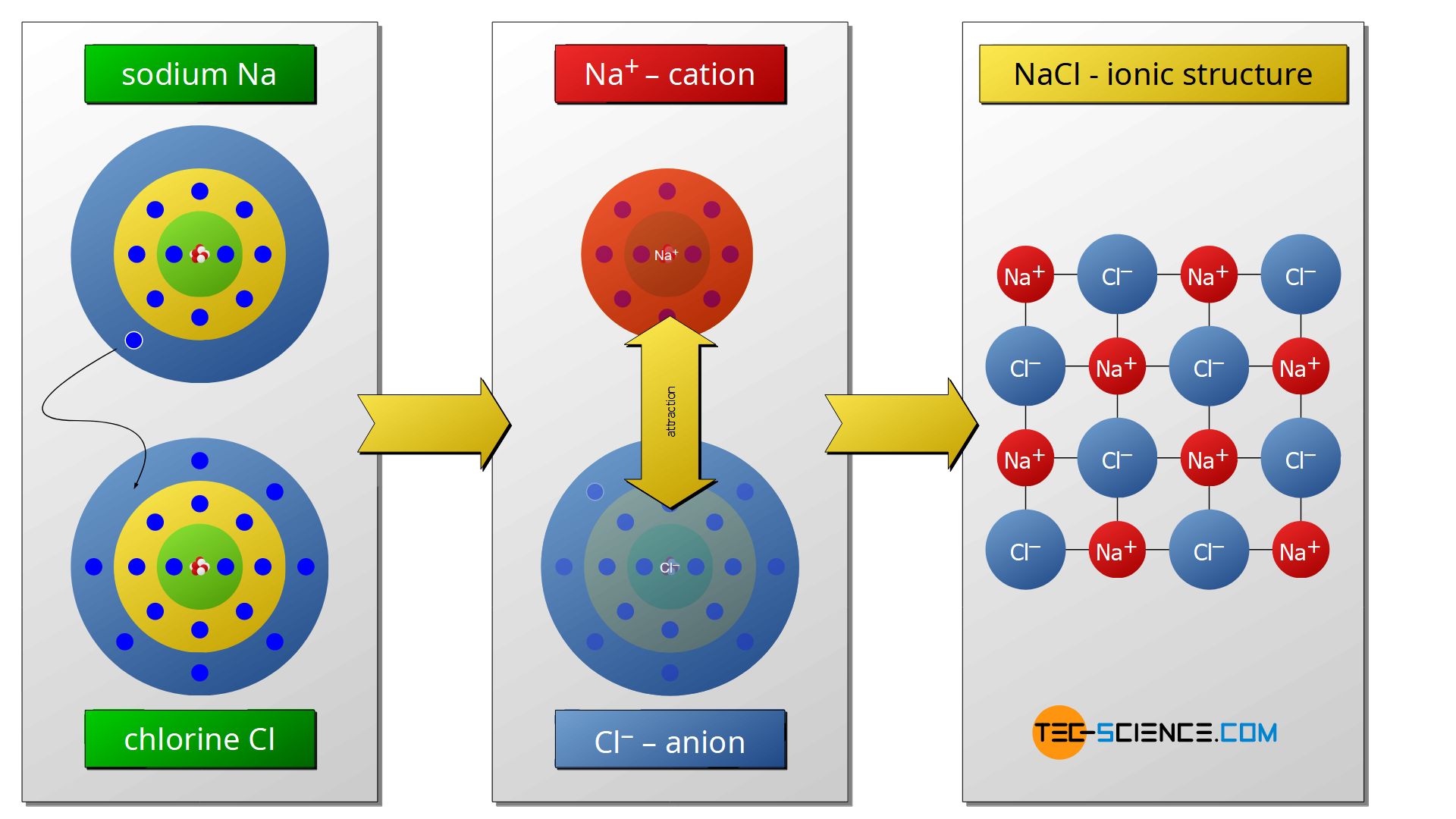
Such crystalline solid compounds of anions and cations are often referred to as salts. A typical example of an ionic compound is therefore common salt, also known as table salt (NaCl). In this compound, the sodium atoms (Na) as alkali metals give off their only valence electrons. The metal atoms thus lose their third M shell, so that on the underlying L shell, the noble gas configuration with eight outer electrons is obtained. The emitted electrons of the sodium atoms are taken up by the nonmetallic chlorine atoms. The chlorine atoms with their seven outer electrons thus now bind eight outer electrons around each other, thus achieving the noble gas configuration. As a result, a crystal structure (ionic lattice structure) ist obtained by the attractive forces between the positive sodium atoms and the negative chlorine atoms.
Salts are ionic compounds consisting of anions and cations!
Basically, the tendency of an atom to bind electrons to itself is particularly great when only a few external electrons are missing to achieve the noble gas configuration. This applies in particular to the elements of the group of halogens with seven valence electrons (e.g. chlorine). Conversely, the tendency to take up electrons is low for those atoms which have only a small number of valence electrons. For these atoms it is usually energetically cheaper to donate the few electrons instead of take up so many. This is especially true for the group of alkali metals whose atoms have only one external electron each (e.g. Na).
Such a more or less pronounced tendency of atoms to attract additional electrons in the binding case is referred to electronegativity. For the above reasons, the electronegativity increases from left to right within a period of the periodic table. This can also be explained by the fact that the number of protons and thus the positive charge of the nucleus increases with increasing atomic number. This increases the atom’s ability to bind electrons as well. The values for the electronegativity of the chemical elements are shown in the figure below (the darker the red the higher the electronegativity).
On the other hand the electronegativity usually decreases from top to bottom within a group. The reason for this is the greater distance of the outermost shell from the atomic nucleus, since with each period a new shell is added. Due to the greater distance, the attractive force between the nucleus and the valence electrons is reduced. Accordingly, the ability of the atoms to attract more electrons decreases. Note that the noble gases can not be assigned electronegativity because they do not bind or tend to donate electrons. The artificially generated elements can as well not be assigned electronegativity for practical reasons, since they are not stable and thus cannot be examined.
The tendency of atoms to attract electrons in the binding case is referred to as electronegativity. In the periodc table the electronegativity usually increases from left to right but decreases from top to bottom!
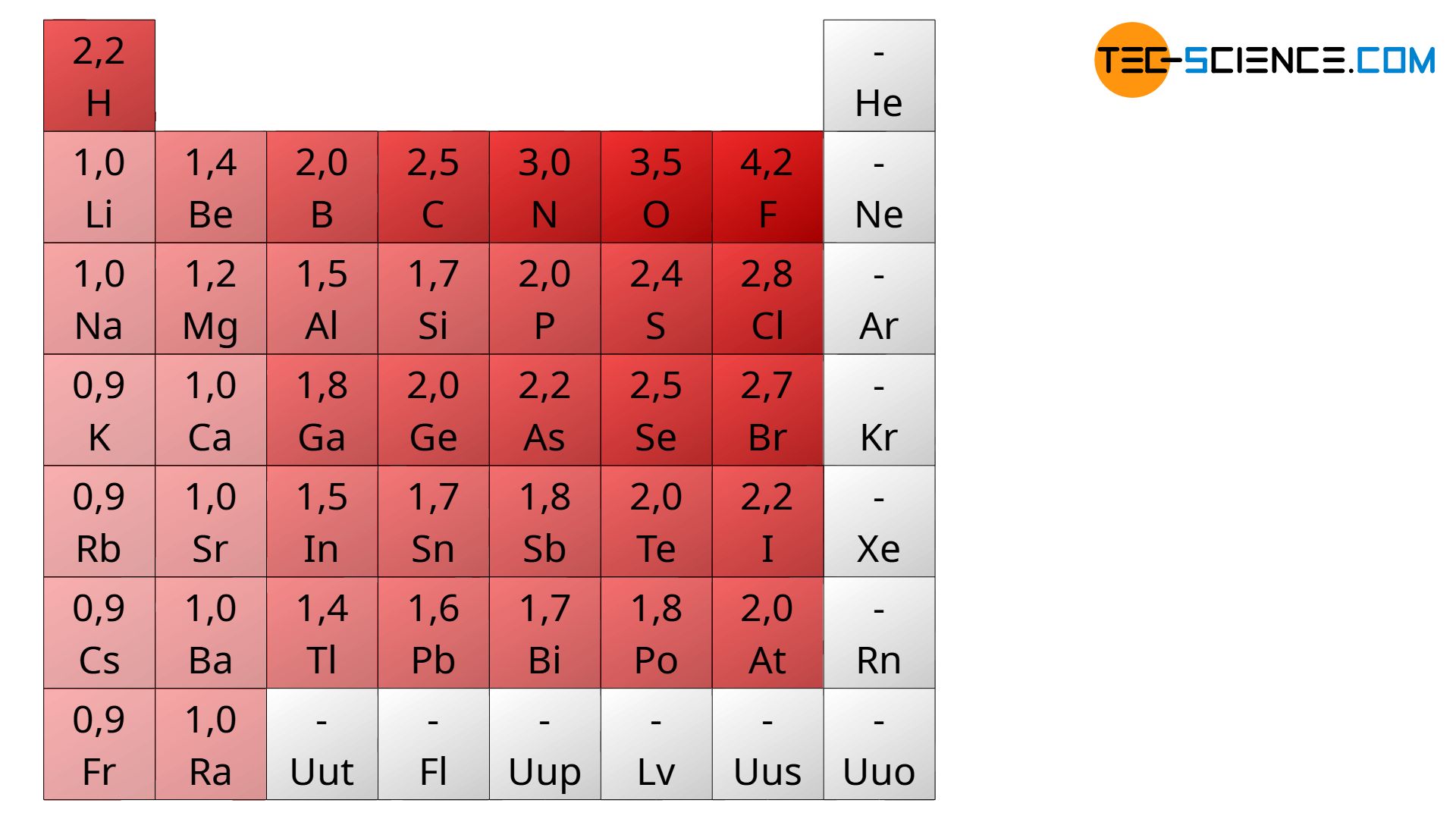
Whether an ionic bond wil be occur or not depends on the property of the atoms involved to release or take up electrons. The non-metal atoms should have the highest possible tendency to take up electrons (high electronegativity), while the metal atoms should tend to electron donation (low electronegativity). For this reason, it can be concluded of their ionic character from the difference of the electronegativity values of the two elements.
If the difference in the electronegativities of two chemical elements is greater than 1.8, then mainly an ionic bond will be present. On the other hand, if the difference is less than 1.8, a covalent bond is predominantly to be expected. In principle, however, there is always a certain amount of covalent behavior in ionic bonding. For example, sodium chloride has an ionic binding character of about 75%. The remaining 25% is accounted for a covalent part.
If the difference in the electronegativities of two chemical elements is greater than 1.8, then mainly an ionic bond will be present!